Organic Industrial processes
__The Contact Process:
__The contact process makes sulphur dioxide – which converts sulphur dioxide into sulphur trioxide (reversible reaction) – which then converts sulphur trioxide into concentrated sulfuric acid.
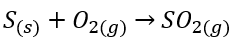
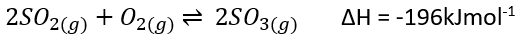

- Converting sulphur trioxide into sulfuric acid can’t be done by simply adding water as the reaction is uncontrollable. Instead, sulphur trioxide is first dissolved in sulfuric acid to produce ==oleum==.
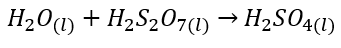
- ==Oleum== can then be reacted safely with water to produce sulfuric acid.
The catalyst used to increase the rate of reaction is ==Vanadium Pentoxide (V2O5)
==Temperature: ==400-450°C
==Pressure: ==>1atm (101kPa) (atmospheric pressure), maximum 5atm
==__The Habor Process:
__The Haber process creates ammonia (NH3) by combining nitrogen from the air with hydrogen derived from methane gas.
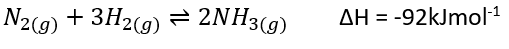
The catalyst used to increase the rate of reaction is ==iron==. It is also used with ==potassium hydroxide (KOH)== which acts as a promoter to increase efficiency.
Pressure: ==200atm (20265kPa)
==Temperature: ==400-450°C