PROTEINS
Protein: From the Greek proteios – “ of first importance”
Naturally occuring unbranched polymer in which the monomer units are amino acids
Proteins account for 15% of a cell’s overall mass.
Unlike lipids and carbohydrates, proteins are not stored, so they must be consumed daily.
Current recommended daily intake for adults is 0.8 grams of protein per kg of body weight (more is needed for children).
Classification based on function
Catalytic proteins - Enzymes (Biochemical Catalyst, helps speed up the biochemical process in the body)
Defense proteins - immunoglobins or antibodies
Transport proteins – hemoglobin, lipoproteins (Helps transfer chemicals in the body)
Messenger proteins – insulin, glucagon
Contractile proteins – actin, myosin
Structural proteins - collagen, α-keratin (e.g skin, hair)
Transmembrane proteins – protein channels
Storage proteins – myoglobin (stores oxygen so that it is readily available)
Regulatory proteins – proteolytic enzymes, zymogens
Nutrient proteins – casein, ovalbumin (Where amino acids come from)
Buffer proteins – hemoglobin (if there are changes in the blood the PH will adjust)
Buffer is the chemical term where the protein absorbs extra acidity so that the PH is maintained.
Blood PH – Ranges from 0 – 14
Neutral is 7, If it is below 7.35 the person is considered to be in acidosis.
Fluid balance proteins – albumin, globulin
Functions of Proteins
Hemoglobin: Transport protein that carries O2 in the blood
Keratin: Fibruos protein in hair, skin, and nails.
Insulin:
Protein hormone synthesized in the pancreas
Controls blood glucose levels
Actin & Myosin: Proteins that control muscle contractions
Ferritin: Protein that stores iron in the liver
Collagen:
Fibruos protein in connective tissue
Found in tendons, bone, cartilage, and blood vessels
Protein Classification by Shape
There are 100,000 different proteins in the human body.
Fibruos proteins: insoluble in water and used for structural purposes (keratin & collagen)
Globular proteins: more or less soluble in water and used for nonstructural purposes
Amino Acids
Are the building blocks of proteins
Contain carboxylic acid and amino groups
Are ionized in solution (soluble in water)
They are ionic compounds (solids – high melting points)
Contain a different side group (R) for each
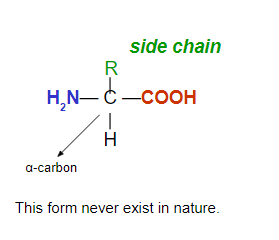
Amino acids are classified as:
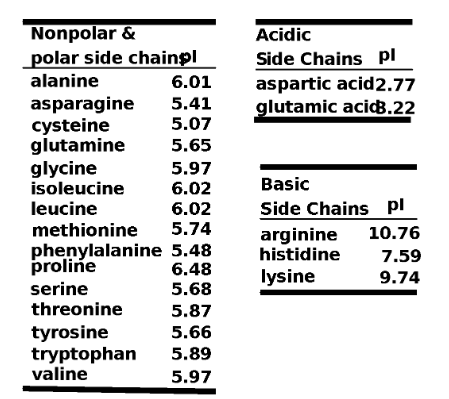
Nonpolar (Neutral) amino acids (hydrophobic) with hydrocarbon (alkyl or aromatic) sides chains.
Polar (Neutral) amino acids (hydrophilic) with polar or ionic side chains.
Polar Acidic amino acids (hydrophilic) with acidic side chains (-COOH).
Polar Basic amino acids (hydrophilic) with –NH2 side chains.
There are only 20 different amino acids in the proteins in humans. They are called a – amino acids.
Humans cannot synthesize 10 of these 20 amino acids
These amino acids must be obtained from a diet (on an almost daily basis)
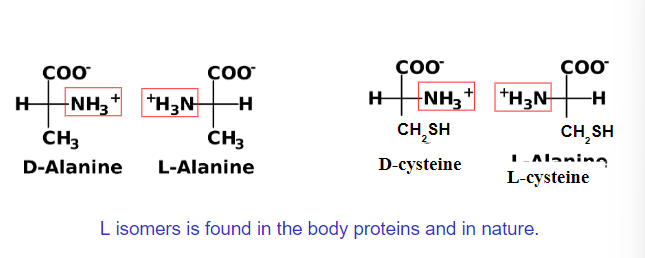
Fischer Projections
All of the a-amino acids are chiral (except glycine)
Four different groups are attached to central carbon
Ionization and pH
The net charge on an amino acid depends on the pH of the solution in which it is dissolved.
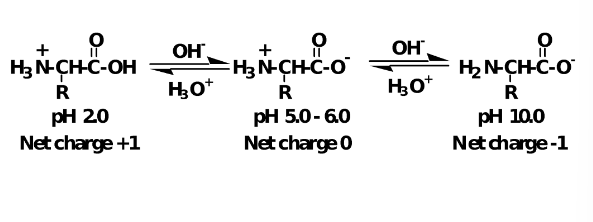
Each amino acid has a fixed and constant pI.
Peptide
A dipeptide forms:
When an amide links two amino acids (Peptide bond).
Between the COO− of one amino acid and the NH3 + of the next amino acid.
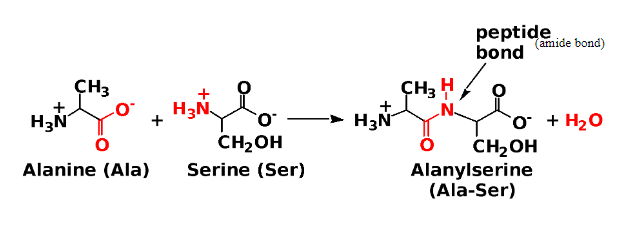
Dipeptide: A molecule containing two amino acids joined by a peptide bond.
Tripeptide: A molecule containing three amino acids joined by peptide bonds.
Polypeptide: A macromolecule containing many amino acids joined by peptide bonds.
Protein: A biological macromolecule containing at least 40 amino acids joined by peptide bonds.
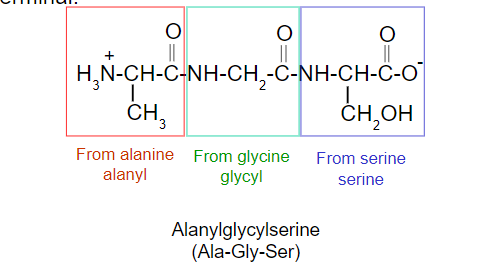
Naming of Peptides
C-terminal amino acid: the amino acid at the end of the chain having the free -COO- group (always written at the right).
N-terminal amino acid: the amino acid at the end of the chain having the free -NH3+ group (always written at the left).
Begin from the N terminal.
Drop “-ine” or “-ic acid” and it is replace by “-yl”.
Give the full name of amino acid at the C terminal.
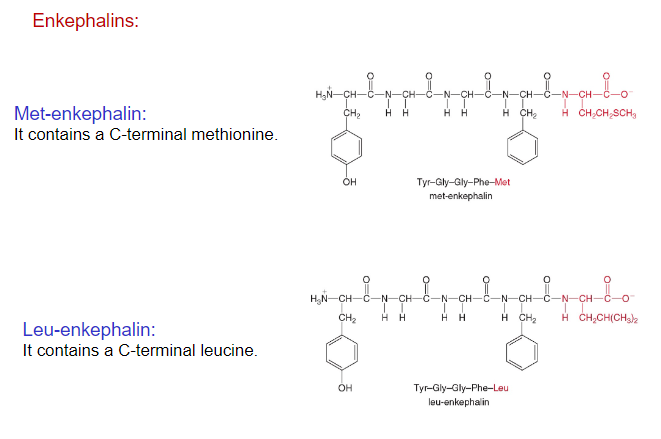
Biologically Active Peptides
Enkephalins, pentapeptides made in the brain, act as pain killers and sedatives by binding to pain receptors.
Addictive drugs morphine and heroin bind to these same pain receptors, thus producing a similar physiological response, though longer lasting.
Enkephalins belong to the family of polypeptides called endorphins (16-31 amino acids), which are known for their pain reducing and mood enhancing effects.
Oxytocin and vasopressin are cyclic nonapeptide hormones, which have identical sequences except for two amino acids.
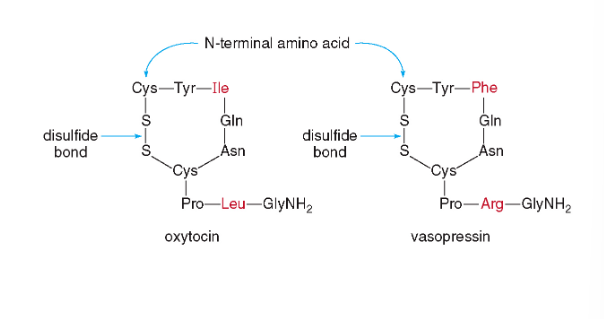
Oxytocin stimulates the contraction of uterine muscles, and signals for milk production; it is often used to induce labor.
Vasopressin, antidiuretic hormone (ADH) targets the kidneys and helps to limit urine production to keep body fluids up during dehydration.
Conantokins are a small family of helical peptides that are derived from the venom of predatory marine snails of the genus Conus.
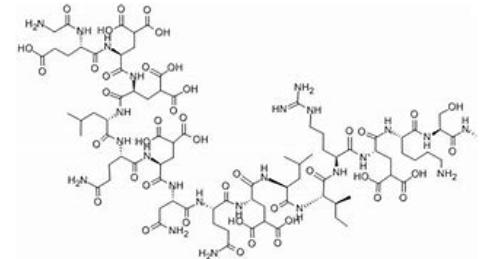
Conantokins are linear conopeptides 17–27 residues in length that contain multiple γ-carboxyglutamate residues in their sequence. Because of the lack of disulfide bonds in the sequence of conantokins, the presence of Gla is important for the formation of a helical structure. The binding of calcium ions to these peptides leads to a conformational change in their structure thought to be important for their bioactivity.104
The therapeutic potential of clinically available NMDA receptor antagonists is currently limited because of the prevalence of undesirable side effects, thought to be a result of a lack of specificity.
Contulakin-G was discovered over 15 years ago as a member of the neurotensin (NT) family from the venom of predatory marine snail, Conus geographus
Contulakin-G is a 16 amino acid peptide
Dr. Lourdes J. Cruz is the National Scientist whose research has contributed to the discovery of these peptides
Structure of Proteins
Primary Structure of Proteins
The order of amino acids held together by peptide bonds.
Each protein in our body has a unique sequence of amino acids.
The backbone of a protein.
All bond angles are 120o, giving the protein a zigzag arrangement.
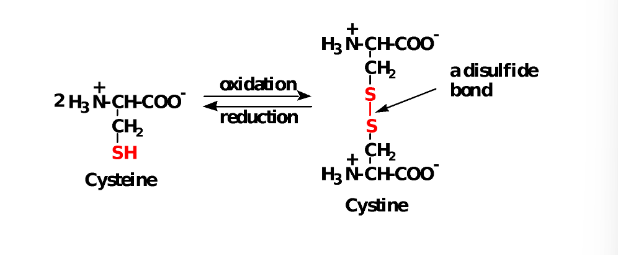
Cysteine
The -SH (sulfhydryl) group of cysteine is easily oxidized to an -S-S- (disulfide).
Primary Structure of Insulin
Is a hormone that regulates the glucose level in the blood.
Was the first amino acid order determined.
Contains of two polypeptide chains linked by disulfide bonds (formed by side chains (R)).
Chain A has 21 amino acids and chain B has 30 amino acids.
Genetic engineers can produce it for treatment of diabetes.
Secondary Structure of Proteins
Describes the way the amino acids next to or near to each other along the polypeptide are arranged in space.

Secondary Structure a-helix
A section of polypeptide chain coils into a rigid spiral.
Held by H bonds between the H of N-H group and the O of C=O of the fourth amino acid down the chain (next turn).
looks like a coiled “telephone cord.”
All R- groups point outward from the helix.
Myosin in muscle and α-Keratin in hair have this arrangement.
Secondary Structure B-pleated sheet
Consists of polypeptide chains (strands) arranged side by side.
Has hydrogen bonds between the peptide chains.
Has R groups above and below the sheet (vertical).
Is typical of fibrous proteins such as silk.
Secondary Structure Triple helix (Superhelix)
Collagen is the most abundant protein.
Three polypeptide chains (three α-helix) woven together.
It is found in connective tissues: bone, teeth, blood vessels, tendons, and cartilage.
Consists of glycine (33%), proline (22%), alanine (12%), and smaller amount of hydroxyproline and hydroxylysine.
High % of glycine allows the chains to lie close to each other.
We need vitamin C to form H-bonding (a special enzyme).
Tertiary Structure
The tertiary structure is determined by attractions and repulsions between the side chains (R) of the amino acids in a polypeptide chain.
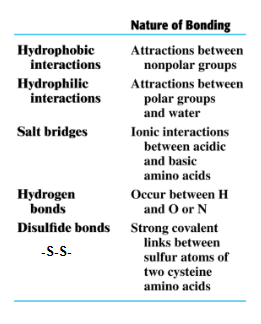
Interactions between side chains of the amino acids fold a protein into a specific three-dimensional shape.
(1)Disulfide (-S-S-)
(2) salt bridge (acid-base)
(3) Hydrophilic (polar)
(4) hydrophobic (nonpolar)
(5) Hydrogen bond
Tertiary Structure
Globular Proteins
Have compact, spherical shape.
Almost soluble in water.
Carry out the work of the cells Synthesis, transport, and metabolism.
Myoglobin
Stores oxygen in muscles.
153 amino acids in a single polypeptide chain (mostly α-helix).
Fibruos Proteins
Have long, thin shape and insoluble in water.
Involve in the structure of cells and tissues.
α-keratin: skin, nail, hair, and bone
β-keratin: feathers of birds
α-keratin: hair, wool, skin, and nails
They are made of two mainly α-helix chains coiled around each other in a superhelix (supercoil).
These coils wind around other coils making larger and stronger structures (like hair).
- α-helix chains bond together by disulfide bond (-S -S-)
- More disulfide bonds, more rigid materials (horns & nails).
Quarternary Structure
Occurs when two or more protein units (polypeptide subunits) combine.
Is stabilized by the same interactions found in tertiary structures (between side chains).
Hemoglobin consists of four polypeptide chains as subunits.
Is a globular protein and transports oxygen in blood (four molecules of O2).
CO is poisonous because it binds 200 times more strongly to the Fe2+ than does O2 (Cells can die from lack of O2).
Conjugated Proteins
They are composed of a protein unit and a nonprotein molecule.
Sickle Cell Hemoglobin
Sickle cell anemia is a disease where a single amino acid of both β subunits is changed from glutamic acid to valine.
A genetic mutation in the DNA sequence that is responsible for synthesis of hemoglobin.
Red blood cells containing these mutated hemoglobin units become elongated and crescent (sickle) shaped (more fragile).
These red blood cells will rupture capillaries, causing pain and inflammation, leading to organ damage, and eventually a painful death.
Summary of Protein Structure
Denaturation
Is a process of destroying a protein by chemical and physical means.
We can destroy secondary, tertiary, or quaternary structure but the primary structure is not affected.
Denaturing agents: heat, acids and bases, organic compounds, heavy metal ions, and mechanical agitation.
Some denaturations are reversible, while others permanently damage the protein.
Heat: H bonds, Hydrophobic interactions
Detergents: H bonds
Acids and bases: Salt bridges, H bonds.
Reducing agents: Disulfide bonds
Heavy metal ions (transition metal ions Pb2+, Hg2+): Disulfide bonds
Alcohols: H bonds, Hydrophilic interactions
Agitation: H bonds, Hydrophobic interactions