foundations and water solutions and buffers
8/29 foundations of biochemistry ch 1 &2

Urey and Miller experiment - supported the hypothesis that life originated spontaneously from inorganic compounds reacting with early earth conditions
first cells were anaerobic → developed ability to photosynthesize
RNA world hypothesis - theory that suggests RNA-based life forms came before DNA-based life forms, and that RNA was the primary carrier of genetic information and metabolic processes in early life on Earth.
cyanobacteria → increase in atmospheric oxygen → mass extinction of anaerobes

bacteria
Inhabits soils, surface, waters, tissues of living or decaying organisms
Properties
Many different shapes
Peptidoglycan layer in cell envelope
Single, circular chromosome
Can contain 1+ plasmids
~15,000 ribosomes
eukarya
Same branch as Archaea
Defined by membrane-bound nucleus
Nuclear envelope
Nucleolus
Membrane-bound organelles
o Mitochondria, rough and smooth Endoplasmic reticulum (ER), Golgi complexes, Peroxisomes, Lysosomes
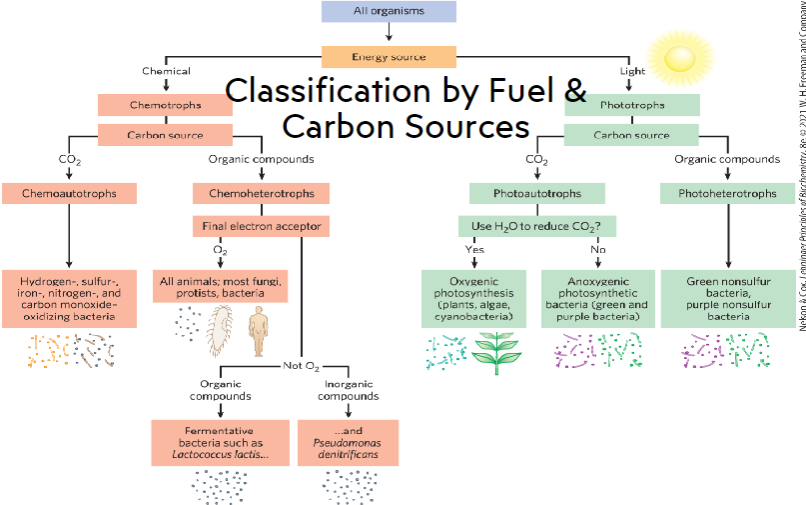
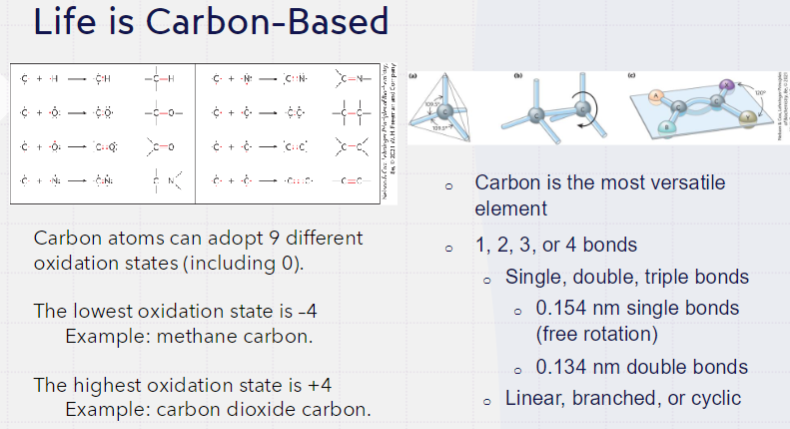
IMPORTANT BIOMACROMOLECULES
proteins, polysaccharides, lipids, nucleic acids
3D structure: configuration
stereoisomers
geometric isomers - two or more coordination compounds which contain the same number and types of atoms, and bonds (i.e., the connectivity between atoms is the same), but which have different spatial arrangements of the atoms.
cis-trans isomers
Cis isomers: Atoms or functional groups are on the same side of a double bond or plane.
Trans isomers: Atoms or functional groups are on opposite sides of a double bond or plane.
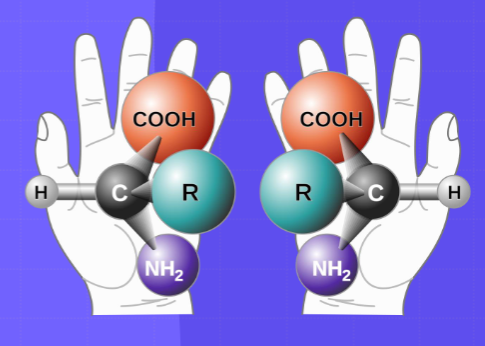
chirality (R vs S) - a geometric characteristic that the structure of an object cannot superimpose with its mirror image
enantiomers - Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other.
diastereomers - are defined as compounds with the same molecular formula and sequence of bonded elements but are non-superimposable non-mirror images.
Chirality matters because it can significantly impact the properties and interactions of molecules, especially in biological systems, as different "handedness" of a molecule can lead to vastly different biological effects, like a drug being effective or toxic depending on its chiral configuration;
L vs D
interactions
substrate-enzyme
hormone receptor
antigen-antibody
Physical foundations (general chemistry review)
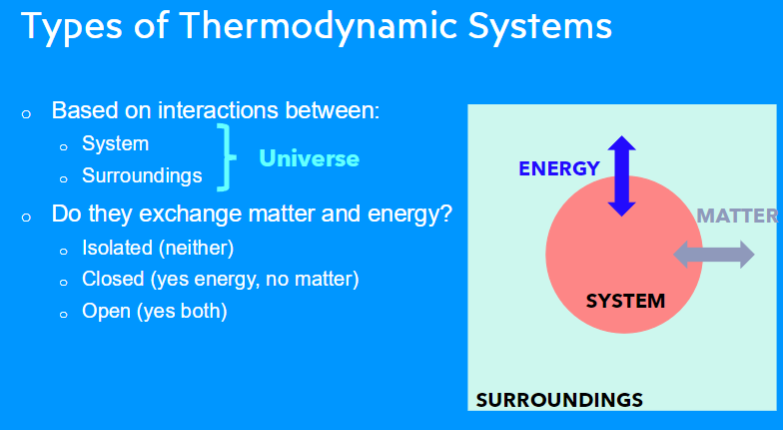
dynamic steady state: a condition within a living organism where the concentrations of molecules and ions inside cells and organs remain relatively constant over time, even though there is a continuous flux of materials coming in and out,
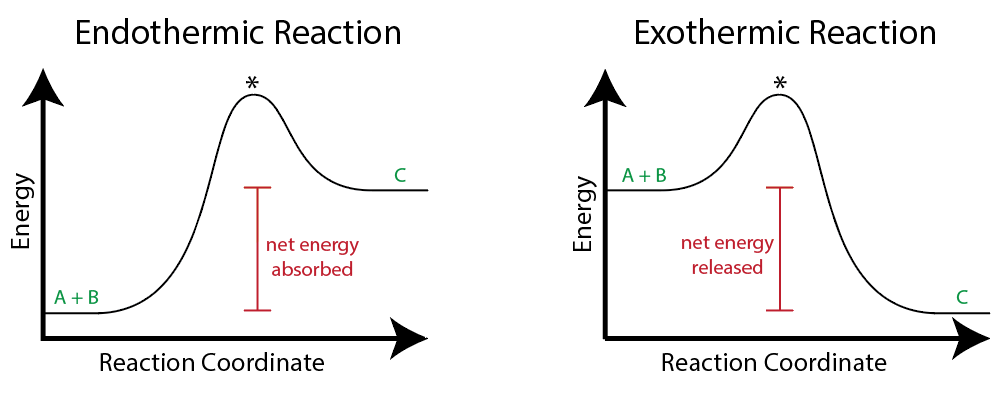
first law of thermodynamics: energy is neither created nor destroyed
energy can change forms

enthalpy = total energy stored in the bonds of molecules
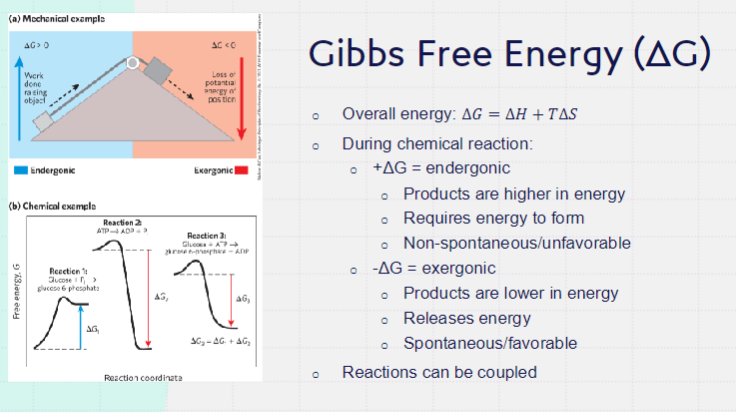
available energy of a substance that can be used in a chemical transformation or reaction
driving force
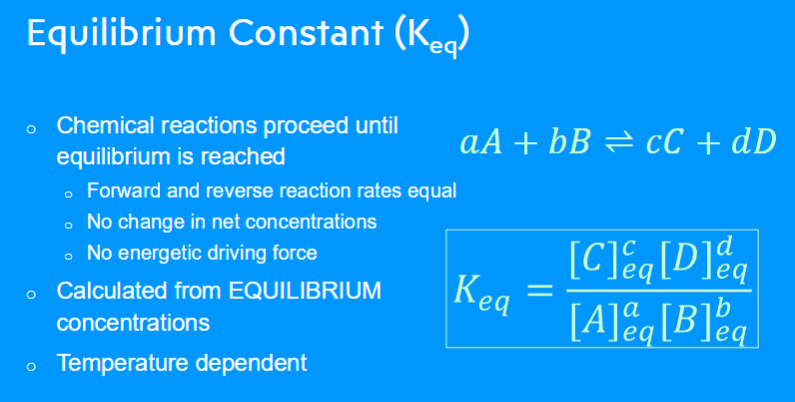
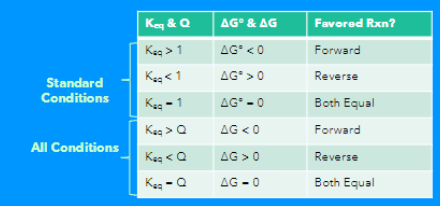
Q = product/reactants (relative amounts of products to reactants present in a chemical reaction at a specific point in time)
enzymes lower the activation energy → increasing rate of reaction
metabolism ??
catabolism
degradative, often involves oxidation, generates energy
anabolism
synthesis, often involves reduction, required energy, heavily regulated
Water solutions and buffers
we are made of mostly water yippee - water is the medium for life!!!
Life evolved in water due to the protection it provides
from UV light.
Organisms typically contain 60–90% water.
most chemical reactions occur in water-based environments
Water is a critical determinant of the structure and function of proteins, nucleic acids, and membranes.
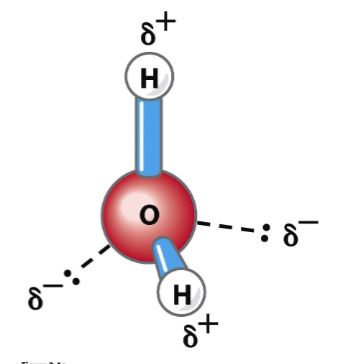
special properties!
can serve as both an H donor and an H acceptor
can have up to 4 H-bonds per water molecule
high boiling point, high melting point, unusually large surface tension
importance of H bonds
Source of unique properties of water, Structure and function of proteins, Structure and function of DNA, Structure and function of polysaccharides, Binding of substrates to enzymes, Binding of hormones to receptors, Matching of mRNA and tRNA
*******
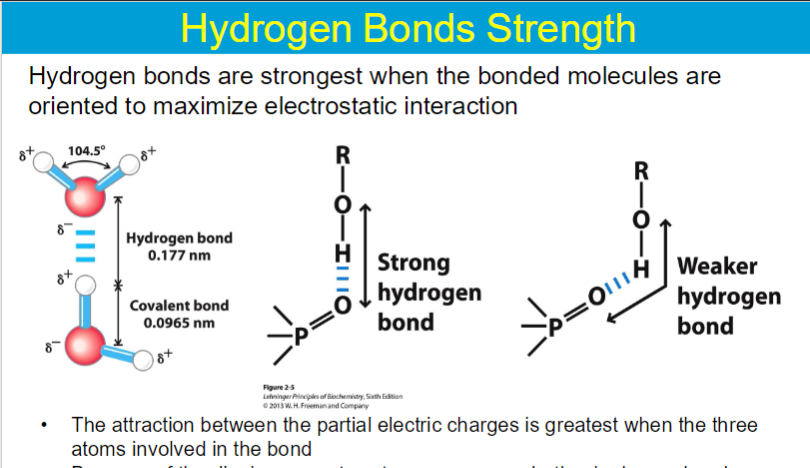
any deviation from this linear alignment, like a smaller angle, reduces the strength of the interaction due to less effective overlap between the partial positive charge on the hydrogen and the lone pair on the acceptor atom.
like dissolves like 😋
lipid molecules have hydrophilic heads and hydrophobic tails
amphipathic - a molecule that has hydrophobic and hydrophilic parts
ions
dielectric constant - measure of its polarity?
higher constant → greater number of dipoles in solvent → better stabilization of ions
dipoles - bond or molecule whose ends have opposite charges
colligative properties (physical changes that result from adding solute to a solvent)
depends on AMOUNT of particles dissolved, not on identity of particle
Vapor pressure lowering
Freezing point depression
Boiling point elevation
OSMOTIC PRESSURE
IONIC compounds have a greater influence because dissociation increases number of particles

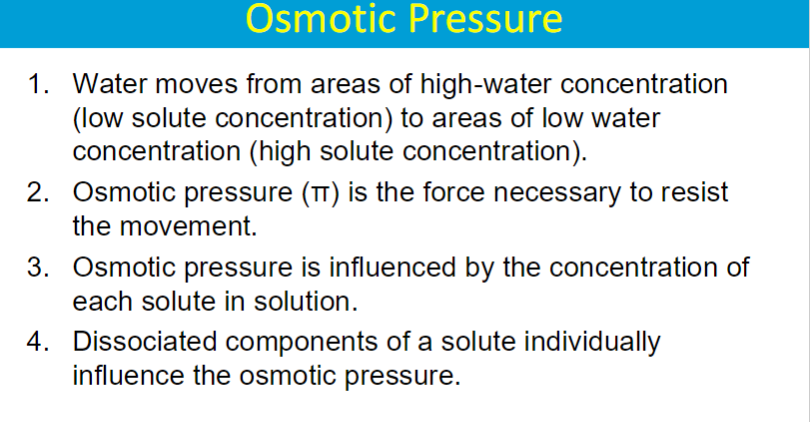
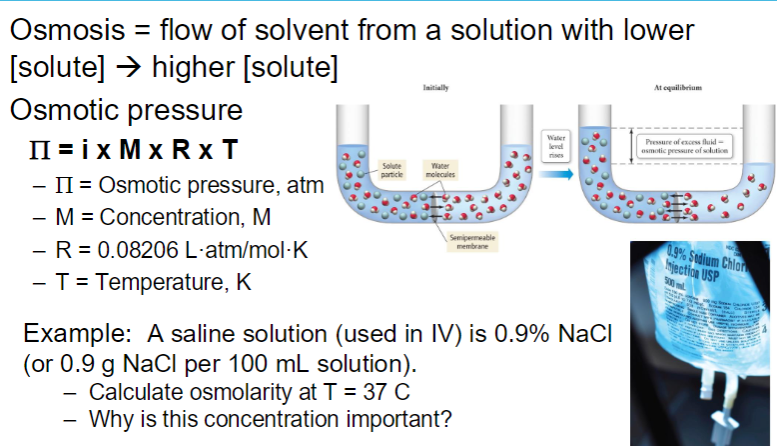
**higher concentration of solute leads to a higher osmotic pressure because osmotic pressure is directly proportional to the solute concetration in a solution
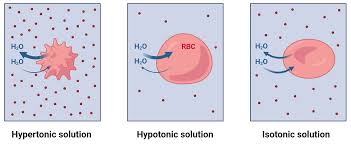
effects of osmotic pressure
Acids and Base
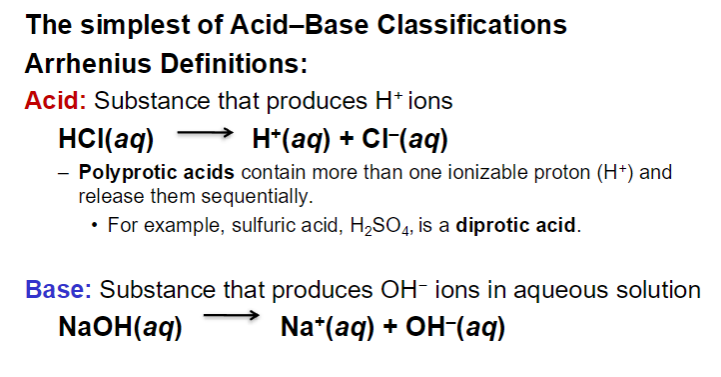
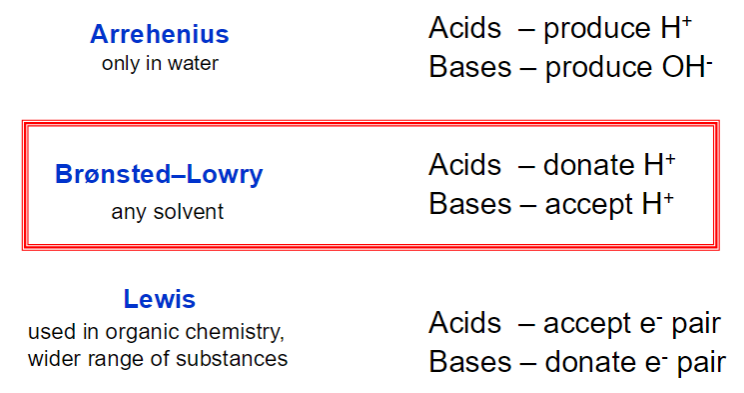
H in a carboxylic acid (COOH) is acidic
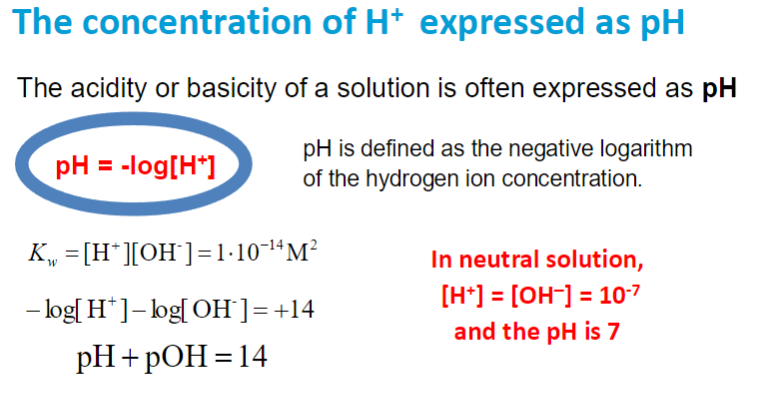
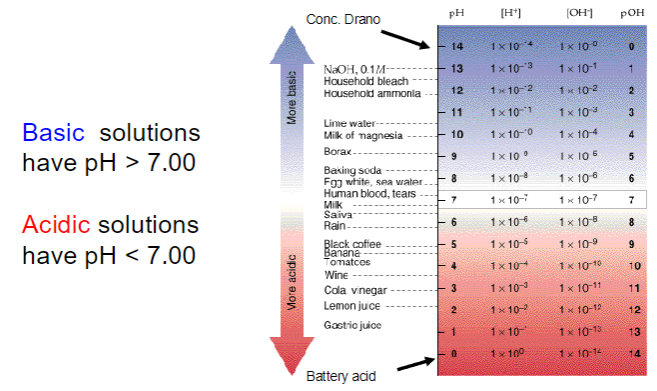
more H+ → more acidic → smaller pH
Buffers and Titrations
buffer system is one that resists changes in pH
2 requirements
composed of a weak acid/weak base conjugate pair
contains a substantial amount of the acid/base and its conjugate
meaning a similar amount
**maximal buffering capacity when the acidic and basic forms are present at equal concentrations i.e. at its pKa
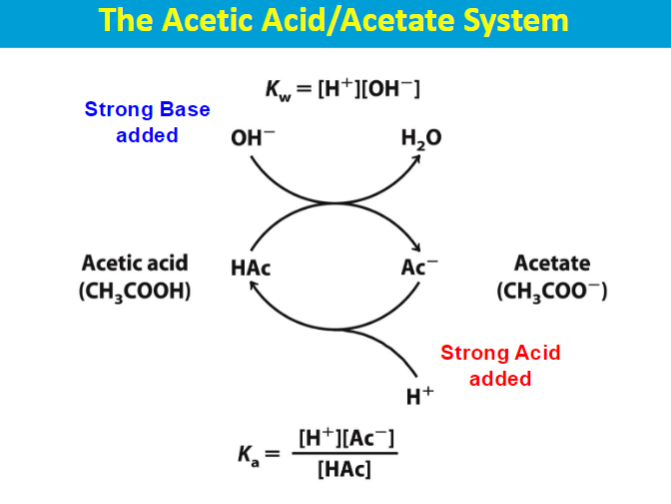
pka - acid dissociation constant
lower pka→ stronger acid
A buffer's capacity is greatest when the pH equals its pKa because at this point, the concentrations of the weak acid and its conjugate base are roughly equal, allowing the buffer to effectively neutralize both added acid and base with minimal change in pH; essentially, the system is optimally balanced to resist pH fluctuations.
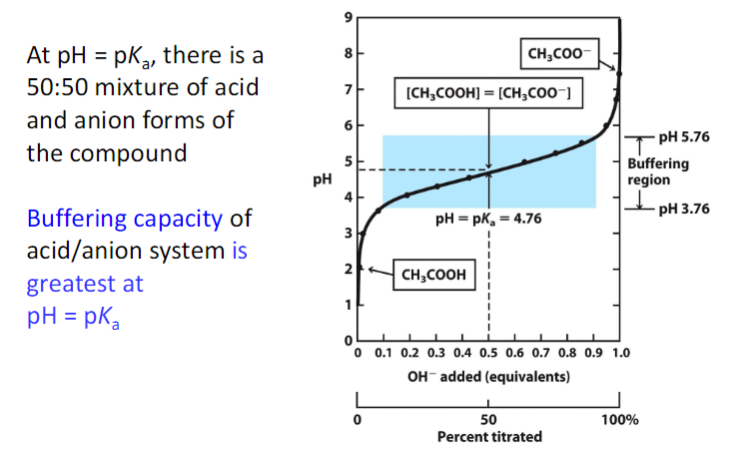
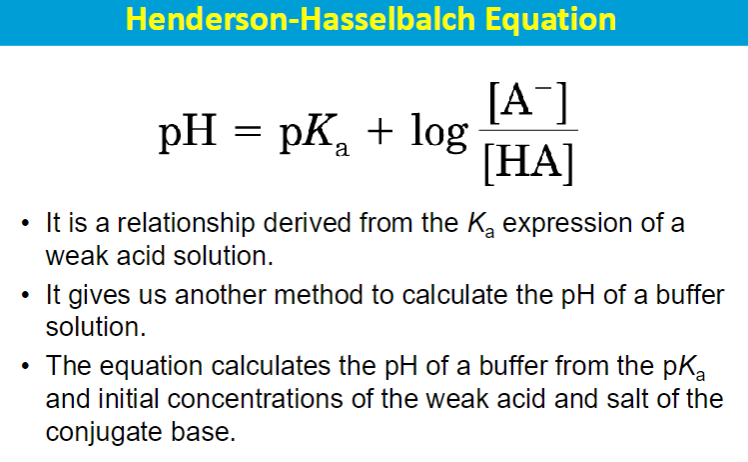
most effective buffering range is typically considered to be pH = pKa ± 1; this means the pH can be one unit above or below the pKa value while still effectively resisting significant pH changes.
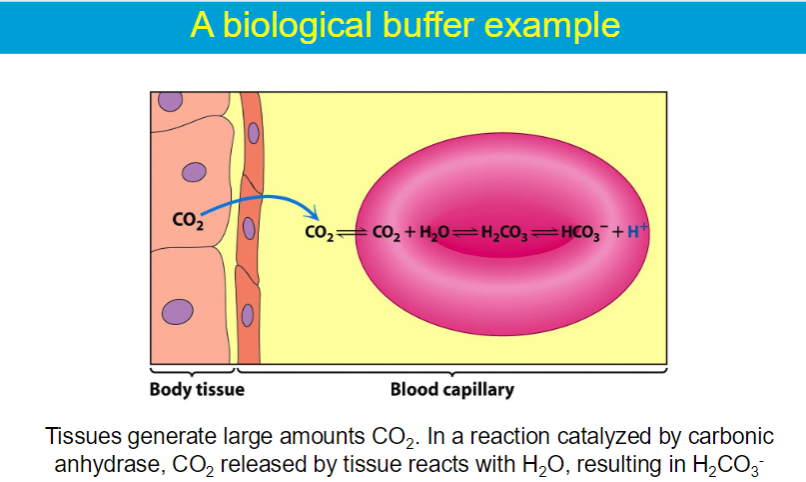
carbonic acid (H2CO3) - weak acid bicarbonate ion (HCO3)- conjugate base
When a small amount of acid enters the bloodstream, the bicarbonate ions react with the hydrogen ions to form carbonic acid, effectively neutralizing the acid. Conversely, when a base enters the blood, carbonic acid can donate a hydrogen ion to neutralize it, forming bicarbonate ions.
helps maintain stable pH level in the blood → ~7.4
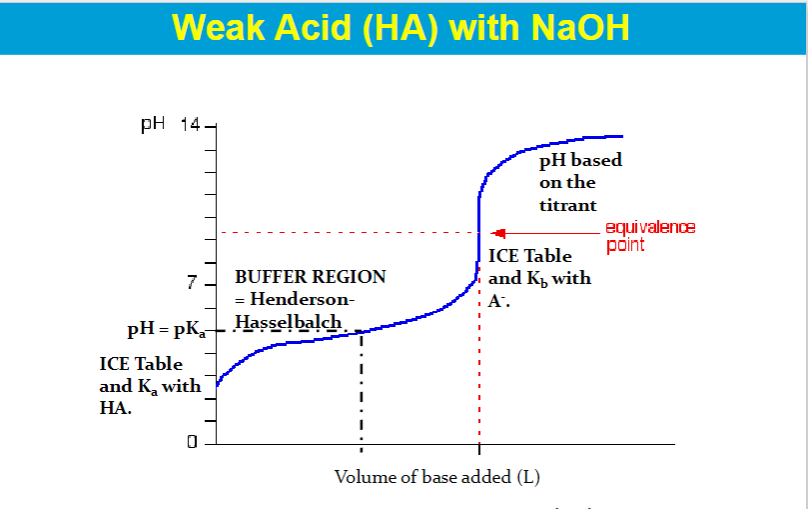
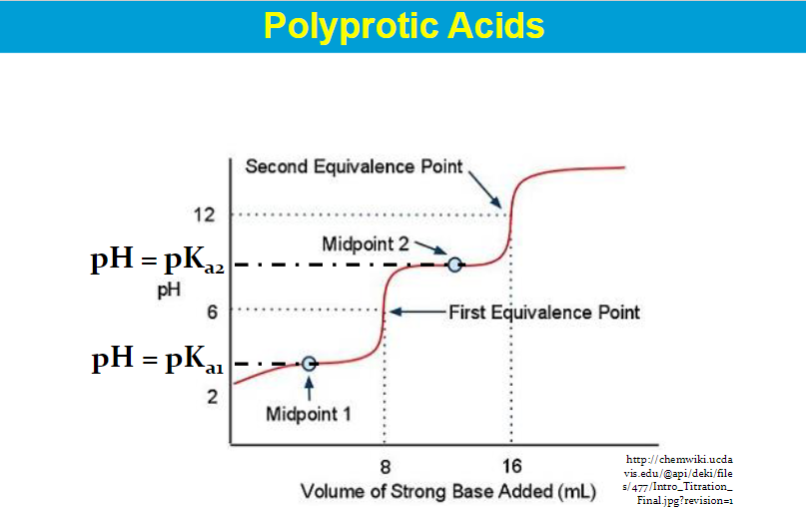
The equivalence point on a titration curve is the point where the amount of titrant added is just enough to neutralize the analyte solution.
It's also the point where the number of moles of titrant added equals the number of moles of the substance being titrated.
"pH=pKa" specifically refers to the point in a titration where the concentration of a weak acid is equal to the concentration of its conjugate base (usually at the "half-equivalence point"), whereas the "equivalence point" is the point where exactly enough acid or base has been added to completely neutralize the other reactant, signifying the end of the chemical reaction in a titration
indicators??