Air and Water (OCR)
Solids, liquids, and gases
The Earth, its atmosphere, and its oceans are made up of solids, liquids, and gases. The particle model describes the arrangement, mobility, and energy of particles within a substance. The model describes the physical properties of substances in solid, liquid, and gaseous states.
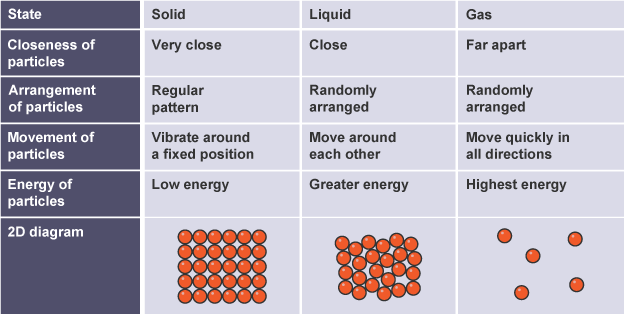
The particles in the diagrams may be atoms, molecules, or ions, depending on the composition.
Explaining physical properties
Solids have a set shape and cannot flow because their particles cannot move from one location to another. They also cannot be compressed since their particles are close together and have no area to go into.
Liquids flow and take the shape of their container because their particles may move around each other. They cannot be compressed because their particles are close together and have no place to move into.
Gases can be compressed because their particles are far apart and have an area to go into, allowing them to flow and fill their container.
Change of State
The figure summarises the most common changes in states.
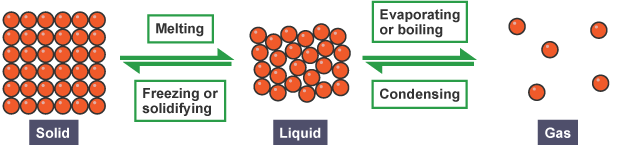
Some compounds can go from solid to gas without first becoming a liquid. This process is known as sublimation. Iodine and solid carbon dioxide ('dry ice') can combine in a process called sublimation.
Changes of state are physical changes. The material remains the same. Only during a chemical shift may a new substance be produced.
Limitations of the particle model: Higher
The particle model depicts particles as inelastic spheres. It makes several simplifications:
It assumes particles are spheres.
It displays spheres as equal in size.
The space between atoms in a gas is tiny enough to fit a diagram on the page, but it should be much greater.
Differences in the forces of attraction between particles are not fully understood.
This means that the particle model's explanations are limited. For example, it cannot explain why certain compounds have various melting and boiling points.
Earth's early atmosphere
The Earth was formed around 4.5 billion years ago. Scientists believe volcanic activity created the early atmosphere.
Composition of the early atmosphere
The early atmosphere likely contained:
A significant amount of carbon dioxide
Water vapour
Small quantities of other gases
Formation of the modern atmosphere throughout time
The oxygen levels have increased
Carbon dioxide levels fell
The earth's oxygen levels
Oxygen levels are considered to have risen considerably around 2.3 billion years ago. Photosynthesis by ancient bacteria may have produced oxygen before this time.
However, oxygen reacted with iron and other things on Earth, so oxygen levels did not grow at first. Only after these chemicals had been oxidized could oxygen levels begin to increase.
Furthermore, early plants and algae began to release oxygen at a higher pace. The amount of oxygen then increased dramatically.
Why did carbon dioxide levels drop?
Carbon dioxide levels are reduced due to processes such as:
Dissolving in the oceans
Plants employ photosynthesis
Fossil fuels form when plants die and their carbon compounds become trapped underground.
Sedimentary rocks formed from the shells of ancient sea animals.
Examples of evidence for the composition of the early atmosphere
Scientists cannot be certain about the makeup of the early atmosphere. Since no measurements can be taken, scientists must rely on indirect evidence from other sources.

How do human actions affect the atmosphere?
An oxidation reaction occurs when oxygen interacts with another chemical. Fuels react fast with oxygen, providing energy to their surroundings through heating and light.
This type of oxidation reaction is referred to as burning or combustion. The byproducts of combustion then enter the atmosphere.
Hydrocarbon fuels
Hydrocarbons are compounds made up of only hydrogen and carbon atoms.
Hydrocarbon fuels can burn completely or incompletely, depending on the amount of oxygen present.
Complete combustion
When there is an adequate supply of air or oxygen, a hydrocarbon fuel will burn completely.
Carbon and hydrogen atoms in the fuel react with oxygen, producing carbon dioxide and water. Energy is released.
In general:
Hydrocarbon + oxygen yields carbon dioxide + water.
Incomplete combustion
Incomplete combustion occurs when the supply of air or oxygen is insufficient. Water is still generated, but carbon monoxide and carbon are the additional byproducts.
Less energy is released compared to complete combustion.
Here is one possible equation for the incomplete combustion of propane.
Propane + oxygen -> CO + carbon + water
C3H8 + 3O2 yields 2CO + C + 4H2O.
It's worth noting that fewer oxygen molecules are required to balance the equation than for complete propane burning.
Soot
Carbon is discharged as fine black particles, which can be seen in smoky flames, and then deposited as soot.
Soot can cause respiratory difficulties and blacken buildings. It has the potential to clog boilers and other appliances, as well as start fires.
Carbon monoxide
Carbon monoxide is a hazardous gas. It is absorbed through the lungs and binds to hemoglobin in red blood cells.
This lowers the blood's capacity to carry oxygen. Carbon monoxide produces drowsiness, and those affected may become unconscious or even die.
Atmospheric pollutants
The combustion of hydrocarbon fuels emits more than just carbon and soot into the atmosphere. Sulfur dioxide and nitrogen oxides may also be created.
Scientists monitor the concentrations of contaminants in the atmosphere and devise strategies to reduce them.
Sulfur dioxide
Many hydrocarbon fuels contain naturally occurring sulfur contaminants. When the fuels are burned, the sulfur oxidizes to produce sulfur dioxide gas.
Sulfur + oxygen yields sulfur dioxide.
S(s) + O2(g) yields SO2(g).
Acid rain is formed when sulfur dioxide dissolves in water in clouds.
Effects of Acid Rain
Acid rain harms both the natural and built environment. For example:
It reacts with metals and materials such as limestone, weakening and destroying buildings and monuments.
Destroys the waxy layer on the leaves of trees, making it more difficult for trees to absorb the minerals they require for healthy growth.
Makes rivers and lakes too acidic to allow some aquatic species to exist.
Oxides of nitrogen
When engines burn fuel, they attain high temperatures. At these high temperatures, nitrogen and oxygen in the air can react to form nitrogen oxides. For example:
Nitrogen + oxygen yields nitrogen monoxide.
N2(g) + O2(g) yields 2NO(g).
Nitrogen oxide gas can be further oxidized in air to form nitrogen dioxide gas (NO2).
NOx refers to the combination of these two nitrogen oxides. They are air pollutants. They can react with other compounds in sunlight, resulting in murky, hazardous fog.
Nitrogen dioxide is harmful. It can result in bronchitis and other lung disorders. It also dissolves in clouds and forms an acidic solution, which leads to acid rain.
Reducing air pollution
Reducing air pollution from power stations
Power plants generate power. Everyone can help to reduce air pollutant emissions from power plants by using less electricity.
Gas scrubbing is a method that can remove sulfur dioxide from waste gases.
Reducing air pollution from vehicles
People who use their cars less may minimize air pollution emissions by, for example:
walking
taking public transportation
Car sharing
cycling
Engineers are working to build more efficient engines for vehicles. This means that the engine uses less gasoline, resulting in reduced air pollution.
A catalytic converter in a car's exhaust converts pollutants like carbon monoxide and nitrogen monoxide into less damaging CO2 and nitrogen.
The adoption of low-sulfur fuels has lowered the amount of sulfur dioxide in automotive emissions.
Reactions and temperature changes
Exothermic and endothermic reactions
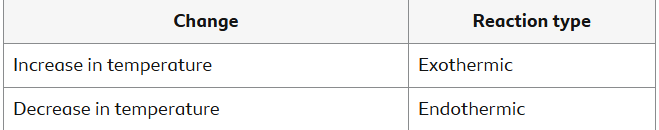
When a fuel burns, it warms the surroundings. Energy is transmitted to the environment. This demonstrates an exothermic reaction.
During some reactions, energy is transferred from the environment. This is referred to as an endothermic reaction. Endothermic reactions allow energy to enter. In exothermic reactions, energy is released.
Temperature Change
For some reactions, the temperature of the reactants at the start and the temperature of the products at the end can be measured separately. Temperature changes show whether the reaction is exothermic or endothermic.
Activation Energy
When a fuel burns, a spark is required to ignite the reaction. A minimal amount of energy is required for a fuel to burn.
The activation energy refers to the minimal amount of energy required for a reaction to occur.
High and low activation energies
For two substances to react, their particles must collide with sufficient energy. The particles in a material have varying energies.
With a low activation energy, many particles will collide and react. The reaction will be quick.
A high activation energy indicates that considerably fewer particles will collide with sufficient energy. The reaction will be gradual.
Reaction Profiles
A reaction profile depicts how the energy levels in the reactants and products fluctuate during a reaction. It contains the activation energy.
The activation energy is represented by a 'hump' in the line, which
Starts from the energy of the reactants.
Is equivalent to the energy differential between the top of the 'hump' and the reactants.
The difference between the energy of the reactants and products represents the overall energy change in a process.
Electricity generated by chemical reactions
Chemical cells
Chemical cells include the common batteries seen in flashlights and mobile phones. Chemical cells come in a variety of designs, each with its own set of reactions.
Chemical cells generate a potential difference, or voltage until the reactants are exhausted. When this occurs, the battery 'goes flat'.
Fuel Cells
Fuel cells function in a different manner than chemical cells. Fuel cells generate a potential difference constantly, as long as they are supplied with:
A fuel
Oxygen (from air)
Hydrogen-oxygen fuel cells
Hydrogen fuel cells could be utilized as an alternative to fossil fuels. More hydrogen fuel cell vehicles could assist in minimizing pollutant emissions.
In a hydrogen-oxygen fuel cell, hydrogen and oxygen are combined to create a potential difference.
The entire process inside the cell is equivalent to the burning of hydrogen. The same product, water, is produced. This means that a hydrogen fuel cell vehicle emits no air pollution.
The general reaction in a hydrogen-oxygen fuel cell is:
Hydrogen + oxygen -> Water.
2H2(g) + O2(g) yields 2H2O(l)
In a hydrogen fuel cell car, the energy produced powers an electric motor rather than an internal combustion engine (a standard car engine).
Hydrogen fuel is often manufactured through electrolysis. Air pollutants are produced during energy generation (in a fossil fuel power station). Although the car itself emits no pollutants, they may be emitted elsewhere.
Evaluate the use of fuel cells.
When analyzing the utilization of fuel cells, the following factors should be considered:
The impact on the environment: emissions from the cell when it is used and emissions as the fuel is produced.
The impact on the user - cost, how well it works, how long the fuel cell can run without refueling, and how it compares to existing technology.
Benefits of employing fuel cells in vehicles
The use of hydrogen-oxygen fuel cells in cars decreases:
CO2 emissions (because water is the only product)
air pollution where the car is being driven.
rely on fossil fuels
Disadvantages of employing fuel cells in vehicles
There are certain disadvantages to employing hydrogen-oxygen fuel cells in vehicles. This includes
Hydrogen is in the gas state at room temperature and pressure, making it difficult to keep in the car.
Fuel cells and electric motors are less durable than gasoline and diesel engines, hence they are not as long-lasting.
Fuel cells are incredibly expensive.
There is currently no countrywide network of hydrogen filling stations.
Some ways of manufacturing hydrogen fuel emit CO2 and other pollutants into the atmosphere.
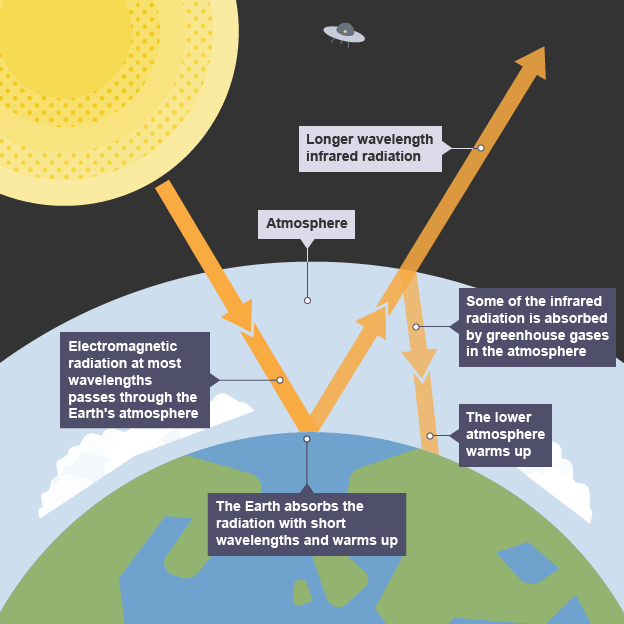
The Greenhouse Effect
Some electromagnetic energy from the Sun travels through the Earth's atmosphere. This comprises infrared, visible, and ultraviolet radiation. This warms the Earth.
The warm Earth emits infrared radiation. Some gases absorb the radiation and then re-emit it in all directions.
Gases that may absorb infrared light are known as greenhouse gases. These include carbon dioxide, water vapor, and methane.
More energy is absorbed than is emitted into space. This causes a warming effect known as the greenhouse effect.
The diagram gives more details about this process:
Changes in climate
Weather varies daily and hourly, specific to certain areas, while climate represents average conditions over long periods.
Observing the weather
Climate is measured by scientists in thousands of locations worldwide. This enables people to recognize trends and patterns in typical circumstances. Measurement uncertainties are taken into consideration by scientists when analyzing data.
Climate data indicates that global temperatures are rising.
Greenhouse gas concentrations in the atmosphere
Over the past 200 years, there has been an increase in the amount of carbon dioxide in the atmosphere.
There is a connection between this and rising fossil fuel use. The atmosphere is filled with carbon dioxide when fossil fuels are burned.
The number of other greenhouse gases in the atmosphere is rising as a result of other human activities. For instance, rice paddies and cow ranching both produce methane emissions.
There is a relationship between rising atmospheric carbon dioxide concentration and changes in world average temperature; as carbon dioxide concentration has climbed, so has the global average temperature.
The connection between the rise in carbon dioxide and the potential temperature increase on Earth can be explained by an intensified greenhouse effect.
Impacts of climate change
Scientists can also use computer models to predict the impacts of climate change.
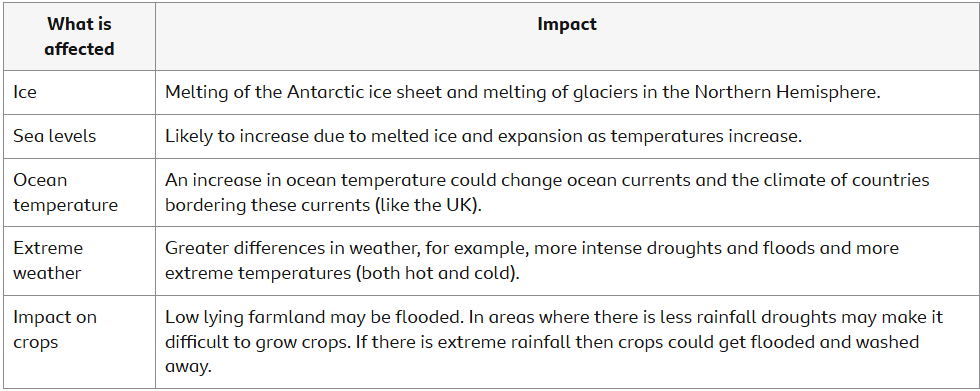
Reducing the effects of climate change
The goal of scientific research is to reduce the effects of climate change. When choosing what to do, both individuals and governments must weigh the advantages and disadvantages to the environment and the populace.
Public regulation, which includes new laws and regulations, can be used to implement certain acts. To have an influence, nevertheless, worldwide agreements and solutions are required due to the enormity of the climate change challenge.
Strategies for reducing carbon dioxide emissions
A system that collects more carbon dioxide than it releases is said to have increased carbon sinks.
Lowering vehicle emissions from automobiles and other sources.
Lowering the emissions from fossil fuel-fueled power plants.
Ways for reducing methane emissions
Reducing emissions from landfills due to biodegradable material degradation
lowering farming's emissions
Decreasing emissions from farming
Drinking water
Water purification
The water's source determines the necessary treatment to turn it into drinkable water.
Surface Water
Rivers, lakes, streams, and ponds have their surface water removed and treated.
The primary processes in this procedure are as follows:
Screening: This step eliminates large, solid objects.
Clarification - allows solids to settle to the bottom.
Filtering - this takes out tiny particles that are floating in the water.
Chlorination - the process of adding chlorine to water to eliminate any germs present.
Groundwater
Underground, water that formerly fell as rain is still trapped thousands of years ago. Some can be accessed by delving into deep subterranean aquifers or by drilling wells.
As the water moved through the sand and rock layers, it was already filtered. The water only has to be chlorinated to eliminate any remaining bacteria before it becomes drinkable.
Wastewater
Some areas of the UK have extremely high water demand. There's a chance that demand will outpace supply during a drought, resulting in a lack of water.
Treatment is one way to make sewage wastewater safe. After that, it can be put back into the river system, where it can subsequently be taken out and used once more.
Screening - removes large solids.
Sedimentation - allows solids to settle to the bottom.
Aeration - adding air and using biological treatment with bacteria. Any organic matter is broken down by the bacteria. Aeration provides the oxygen needed by the bacteria.
Final settlement - allows any remaining very fine particles to settle before the water is returned to the river.
Chlorine testing
Reddish-blue litmus paper turns white when exposed to chlorine gas.
When working with chlorine gas, always utilize a fume cupboard because the gas is toxic.
Water from saltwater
Some parts of the world have limited freshwater resources. It is feasible to convert saltwater into drinkable water if the location is close to the coast.
Desalination is the technique used to get rid of the salt. Water vapor and dissolved salt are separated using distillation:
the water is evaporated
the water vapor is then condensed
A novel method of removing the salt in the newest desalination facilities is membrane filtration. These employ extremely thin membranes. The dissolved salts cannot pass through the membranes, but water molecules may.
Chlorination
Chlorine can eliminate all germs from water. Chlorine is poisonous, though, so using it comes with certain hazards.
Because of this, chlorinating water is governed by laws and frequently prohibited in many nations.
Chlorination is still not utilized in certain regions of the world. Higher risks of waterborne illnesses result from this. Most nations hold the view that chlorination has more advantages than disadvantages.