CHAPTER 4_Biological molecules
Page 1: Biological Molecules
Overview
Biological molecules are essential for life and play a crucial role in cellular functions.
Page 2: Chemical Elements
Categories of Biological Molecules
Most biological molecules fall into three primary categories:
Molecule | Chemical element |
Carbohydrates | Composed of carbon, oxygen, and hydrogen. |
Proteins | Contain carbon, oxygen, hydrogen, and nitrogen; may also include sulfur in small amounts. |
Lipids | Composed of carbon, oxygen, and hydrogen. |
Page 3: Large Molecules from Smaller Molecules
Carbohydrates
Carbohydrates consist of long chains of simple sugars.
Types of Carbohydrates:
Monosaccharides:
e.g., Glucose (simple sugar).
Disaccharides:
e.g., Maltose (formed by joining 2 glucose molecules).
Polysaccharides:
e.g., Starch, glycogen, and cellulose (formed by many glucose molecules).
Page 4: Fats (Lipids)
Structure of Fats
Most dietary fats are triglycerides, which consist of:
→ 1 Glycerol molecule + 3 Fatty Acid chains.
(Fatty acids vary in size and structure)
Lipids are divided into two categories:
Fats → Solid at room temperature.
Oils → Liquid at room temperature.
Page 5: Proteins
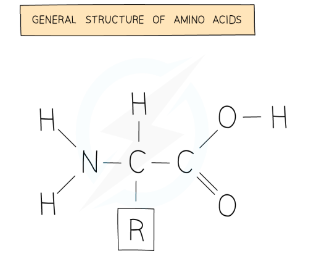
Structure of Proteins
Composed of long chains of amino acids (around 20 different types).
Each amino acid consists of a similar basic structure but has a unique 'R' group.
Small differences in amino acid sequence yield different proteins.
TESTS/ INVESTIGATIONS
Page 6: Food Tests - Testing for Glucose
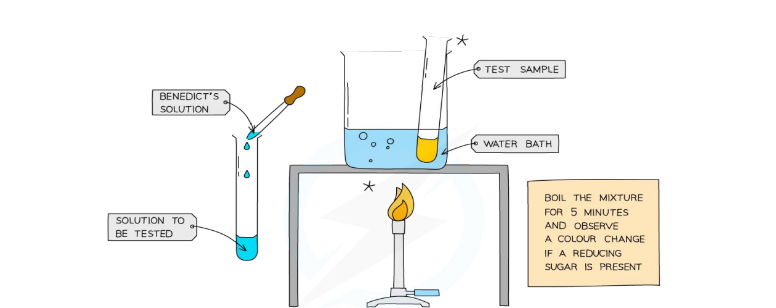
Glucose Test Procedure
Materials: Benedict's solution, food sample, water bath.
Method:
Add Benedict's solution to the food sample in a test tube.
Heat at 60-70°C in a water bath for 5 minutes.
Observe the color change.
→ Benedicts solution + Glucose = to orange or brick red
Result:
Positive test shows a color change from blue to orange or brick red.
Negative test: remains blue, indicating the absence of reducing sugars.
Safety Precautions: Handle test tube with tongs, wear safety goggles, use heatproof gloves.
Page 7: Test for Starch Using Iodine
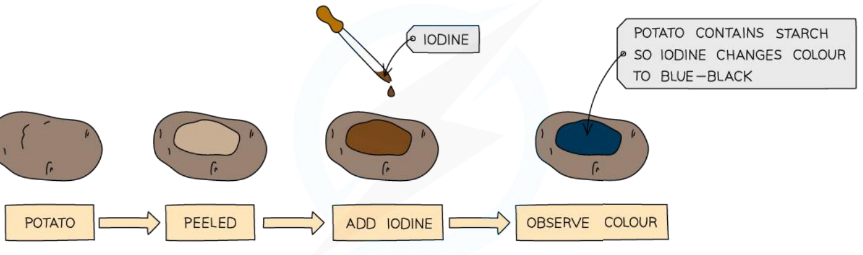
Iodine Test Procedure
Materials: Iodine food sample.
Method:
Add Iodine solution to the food sample.
Observe the color change.
→ Starch + Iodine = Blue-black color change.
Result:
Positive test shows a color change from previous colour to blue-black.
Negative Test: Iodine does not change color with maltose molecules.
Example: Potato contains starch, turning iodine blue-black.
Page 9: Test for Protein
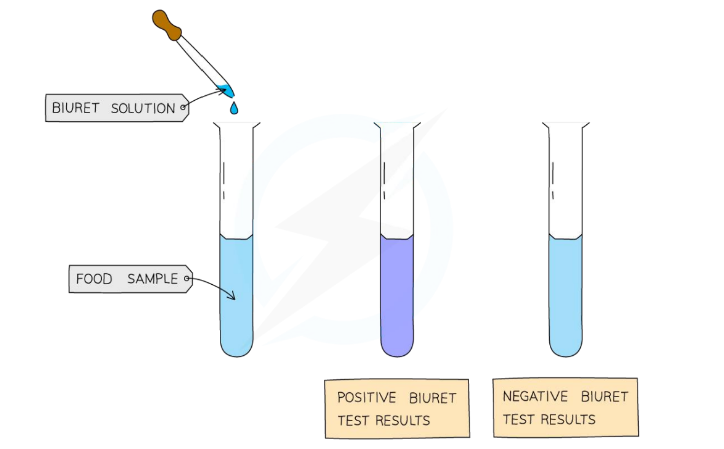
Procedure for Protein Testing
Materials: Biuret solution, food sample, test tube.
Method:
Add Biuret solution to food sample, in the test tube.
Mix gently
Observe the color change.
→ biuret solution + protein = colour change to violet/ purple
Result: Positive test shows a color change from blue to violet/purple.
Negative test: remains blue, indicating the absence of proteins in the food sample.
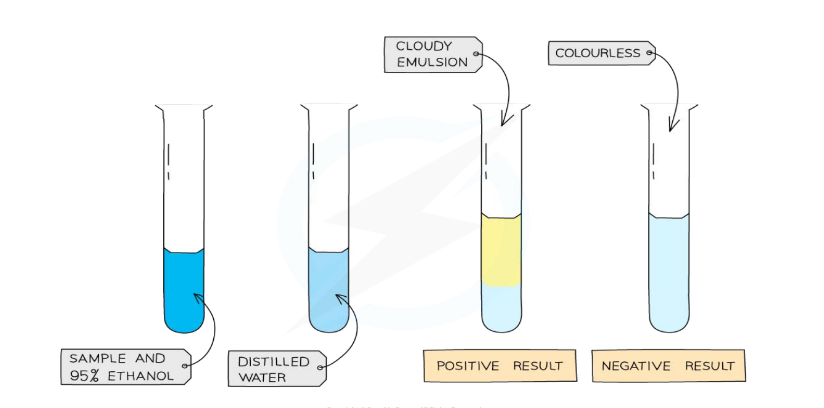
Page 10: Test for Lipids
Lipid Testing Steps
Materials: 2cm³ ethanol, food sample, testing tube.
Method:
Mix food sample with 2cm³ ethanol and shake.
Add an equal volume of cold water.
Observe the color change.
→ ethanol + fats = cloudy white emulsion
Result:
Positive test shows a color change from previous colour to cloudy emulsion.
Negative Test: No color change occurs, indicating the absence of fats in the sample.
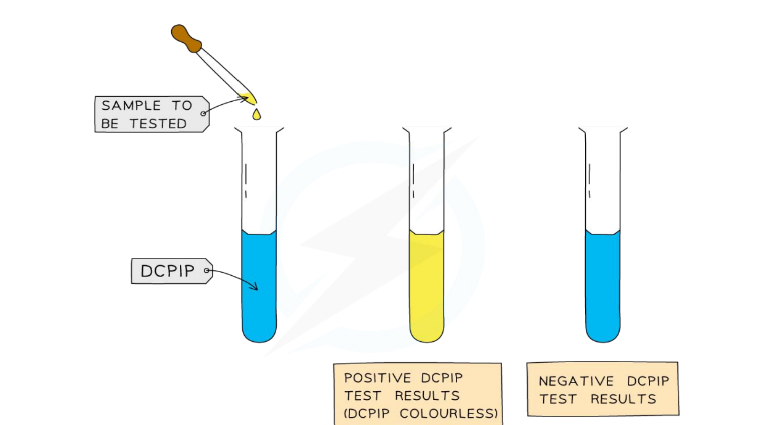
Page 11: Test for Vitamin C
Vitamin C Testing Method
Materials: 1cm³ DCPIP, food sample solution, testing tube.
Method:
Add 1cm³ of DCPIP to a test tube.
Add a small amount of food sample (as a solution).
Observe the color change.
→ DCPIP + Vitamin C = loss of colour
Result:
Positive test shows blue dye color disappears turning colorless.
Negative Test: Colour remains blue.
Page 12: Exam Tip for Food Tests
Important Examination Advice
When describing food tests, mention the starting color and the end color resulting from a positive test.
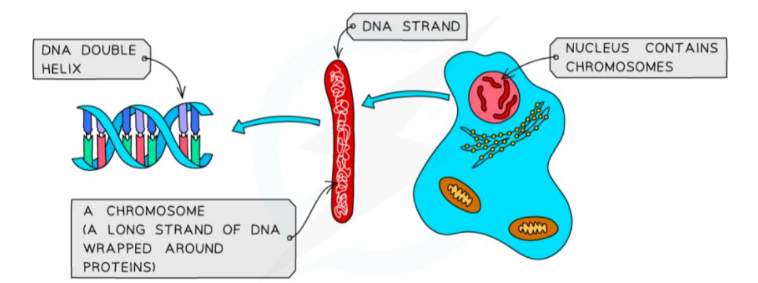
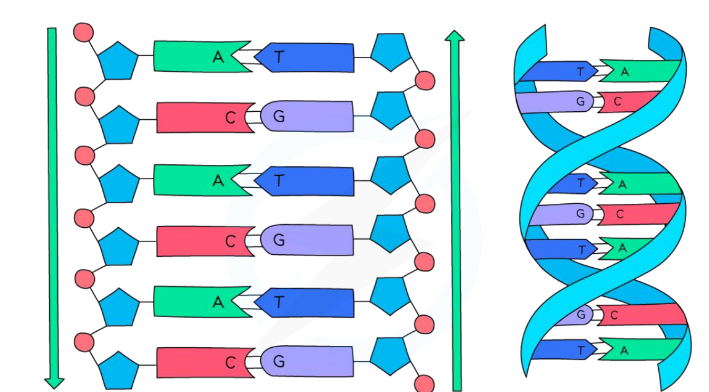
Page 13: Structure of DNA Molecule
DNA Overview
DNA (contains instructions for the growth and development of organisms).
Consists of two strands of DNA in a double helix formation.
Individual units of DNA are called nucleotides.
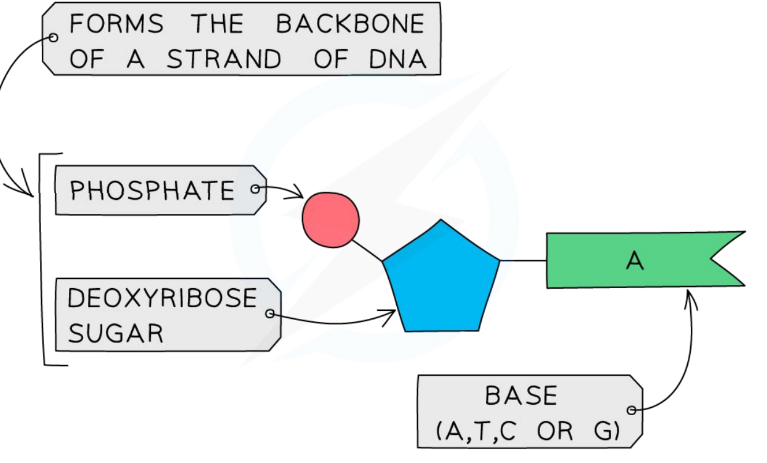
Page 14: Nucleotide Structure
Components of a Nucleotide
Each nucleotide contains:
Backbone of DNA strand: Phosphate group.
Backbone of DNA strand: Deoxyribose sugar.
Base: A nitrogenous base (A, T, C, or G).
Page 15: Base Pairing
Base Pair Specifications
Base pairing rules:
Adenine pairs with Thymine (A-T).
Cytosine pairs with Guanine (C-G).

Page 16: Structure of DNA-Strands
DNA-Backbone and Base Pair Interaction
The sugar-phosphate backbone looks like the sides of a ladder.
Base pairs connect to form the rungs of the ladder.
Gene Definition: Gene is a sequence of bases, not base pairs.
Page 17: Gene Composition
Key Points on Genes
Each DNA strand is separate and holds genetic information as sequences of bases.
DNA helix structure is stabilized by hydrogen bonds.
Exam Tip: Remember base pairings (A-T, C-G)