Biology Chapter 2
The Chemical Context of Life
Matter
Matter is anything that takes up space and has mass.
Matter exits is many forms; the most basic are solid, liquid, and gas. Matter is made up of elements and compounds.
Elements and Compounds
An element is a substance that cannot be broken down by chemical reactions.
Scientists have recognized 92 elements that occur in nature. Some examples are oxygen, gold, carbon, and sodium.
A compound is a substance that consists of two or more different elements in a fixed ratio.
Ex. Sodium chloride (NaCl)
composed of elements sodium and chloride in a 1:1 ratio
Ex. Water (H2O)
composed of elements hydrogen and oxygen in a 2:1 ratio
A compound has properties that are different from the elements it is made up of. These properties are called emergent properties.

The Elements of Life
There are 92 natural elements and 20-25% of them are essential elements that organisms need to survive.
Essential elements are similar between species, but different organisms need numbers of elements to survive. For example, humans have 25 essential elements, while plants only have 17.
Four elements: oxygen, carbon, hydrogen, and nitrogen make up 96% of living matter. Calcium, phosphorus, potassium, sulfur, and a few other elements make up most of the other 4%.
Trace elements are elements that are crucial for life but are only needed in very small amounts.
These include iron and iodine (for humans).
Some naturally occurring elements are toxic to organisms (ex. arsenic).
Atoms
Elements are made up of atoms. Each element is made up of one type of atom. (ex. carbon is made up of carbon atoms.)
An atom is the smallest unit of matter that still retains the property of the element.
Subatomic Particles
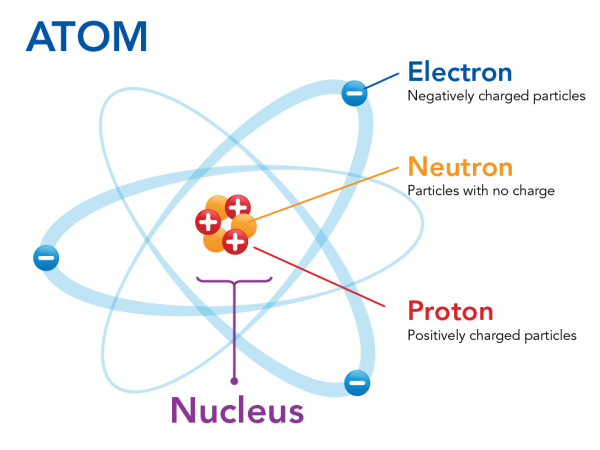
Even though the atom is the smallest unit that retains its element properties, atoms are made up of even smaller subatomic particles. There are three main subatomic particles: protons, electrons, and neutrons.
Protons and electrons are electrically charged: protons have one unit of positive charge, while electrons have one unit of negative charge. Neutrons on the other hand are neutrally charged.
Protons and neutrons are packed together tightly, making up the atomic nucleus. The protons give the nucleus a positive charge. Around the nucleus, electrons move rapidly producing a “cloud” of negative charge. Because of the attraction between the opposite charges, the electrons stay near the nucleus.
Neutrons and protons are almost identical in mass. They are measured in Daltons (which is the same as the atomic mass unit). Neutrons and protons are close to one Dalton, but electrons have such a miniscule mass in comparison that we can ignore them when calculating mass.
Atomic Number and Atomic Mass
The difference between different elements is the number of protons in its atom. (ex. a carbon atom has 6 protons). This unique number of protons is called the atomic number.
Unless otherwise indicated, an atom has a neutral charge, meaning the number of protons should equal the number of electrons in an atom.
The mass number is the number of protons + neutrons in an atom. So, the mass number minus the atomic number gives you the number of neutrons.
Isotopes
All atoms of a given element have the same number of protons; however, they can have a different number of neutrons. These atoms with a different number of neutrons are called isotopes.
ex. carbon has three isotopes: the most common is carbon 12 with 6 protons and 6 neutrons, but there is also carbon 13 (7 neutrons) and carbon 14 (8 neutrons).
Carbon 12 and 13 are stable isotopes while carbon 14 is a radioactive isotope.
A radioactive isotope is an isotope that’s nucleus decays spontaneously giving off particles and energy.
Radioactive decay leads to a change in the number of protons, changing the element of the atom.
ex. When a carbon 14 atom decays, a neutron decays into a proton transforming the atom into a nitrogen 14 atom.
Radioactive Tracers
Radioactive isotopes can be used as tracers in the field of medicine.
Cells use the radioactive atoms of an element, the same way they would use the nonradioactive atoms. Radioactive isotopes are added in the biologically active molecules and then used as tracers to track atoms during metabolism.
ex. To diagnose certain kidney disorders, small amounts of radioactive substances are injected into the blood. The tracer molecules are then analyzed when excreted in urine.
Although radioactive isotopes are incredibly useful in biological research and medicine, they do pose threats such as damaging cellular molecules or through radioactive fallouts from nuclear accidents.
Radiometric Dating
Researchers can study the radioactive decay of fossils to understand how old they are.
The half-life or a radioactive isotope is 50% of the time it takes for it to decay. Radioactive isotopes have half-lives that by any environmental variables. Scientists can calculate many half-lives have passed since the organism was fossilized to understand how old the fossil is.
The Energy Levels of Electrons
Of the three subatomic particles, only electrons are involved in the chemical reactions between atoms.
An atom’s electrons vary in the amount of energy that they possess. Negatively charged electrons are attracted to the positively charged nucleus. The electrons farthest away from the nucleus possess the greatest amount of energy (potential energy).
Electrons are found on different electron shells. The first shell is closest to the nucleus, so it has the least amount of energy, while the farther the shell is from the nucleus, the greater amount of energy it has.
Electron Distribution
The chemical behavior of an atom depends on the distribution of its electrons.
On the periodic table the elements are arranged in rows depending on how many electrons shells they have.
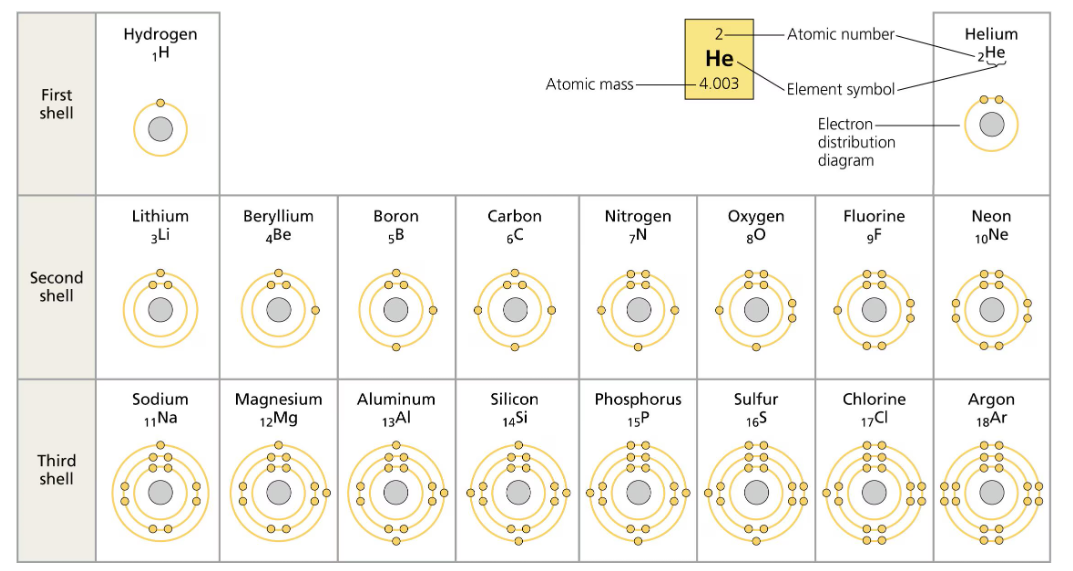
The first electron shell can only hold up to two electrons, which is why hydrogen and helium are the only elements in the first row. The next shells can each hold up to eight electrons.
The outermost shell of the atoms is called the valence shell, and the electrons on it are called the valence electrons. The chemical behavior of the atom is mostly dependent on these valence electrons. The number valence electrons increase as we go to the right of the period table.
An atom with a complete valence shell is unreactive or inert. The unreactive electrons are on the right most column of the periodic table.
Electron Orbitals
The three-dimensional space where an electron stays 90% of the time is called an orbital. No more than two electrons can be in a single orbital. The first electron shell only has 1 orbital. The next electron shells each have 4 orbitals.
Chemical bonding arises from the electrons in the orbitals being unpaired.
Chemical Bonding
Atoms with incomplete valence shells can interact with other atoms to complete their valence shells. There are two ways in which an atom can do this: by sharing or transferring their valence electrons.
Chemical bonds are the attractions between two atoms that result either from sharing valence electrons, or because of their opposite charges. Bonded atoms gain complete valence shells.
Covalent Bonds
A covalent bond is formed by two atoms sharing a pair of valence electrons.
ex. Each hydrogen bond has 1 electron and needs 2 to complete its valence shell. So, the two hydrogen atoms will bond together, and each share their electrons so they both can complete their valence shells.

Two or more atoms held together by covalent bonds results in the formation of a molecule (in this case, a hydrogen molecule).
A molecule can be written using either a subscript as in H2 or with a line showing the bond like this: H — H
A double bond is when two atoms are share two pairs of electrons in two covalent bonds. An example of a double bond an oxygen molecule.
A double bond is written using two lines like this: O = O.
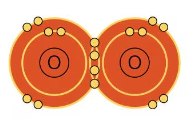
The molecules H2 and O2 are pure elements rather than compounds because they are made up of only one type of element. Water however is H2O, so it is a compound.
Covalent bond examples:
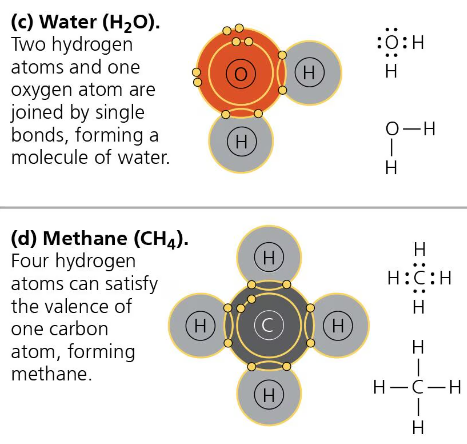
Carbon has 4 unpaired electrons on its valence shell which is important because that means it can bond with for atoms at the same time.
Electronegativity
Depending on the element, atoms in a covalent bond may have a greater attraction to the electrons that they share. Electronegativity is the attraction a given atom has for the electrons being shared in the covalent bond. The more electronegative an atom is, the more it pulls the electrons it shares to itself.
In a covalent bond, the two atoms have the same electronegativity so there is no “tug-of-war” between the two atoms. Such a bond is called a non-polar covalent bond.
ex. an oxygen molecule or hydrogen molecule
However, when an atom is bonded to another atom with a greater electronegativity, the electrons of the bond are not equally shared. This is called a polar covalent bond.
ex. the bonds between hydrogen and oxygen in water
Oxygen is a very electronegative element. Because it was a greater pull on the shared electrons, it takes on a slightly negative charge.
in water, when bonded to oxygen, hydrogen would take on a slightly positive charge.
However, oxygen and hydrogen are NOT ions because they are not completely transferring or taking the electrons.
In the four essential elements (hydrogen, carbon, oxygen, and nitrogen), Oxygen is the most electronegative with nitrogen the second most electronegative. Carbon and hydrogen have the least electronegativity of those four and their electronegativity is relatively the same.
Ionic Bonds
In some cases, the electronegativity difference is so great that one atom completely takes away a valence electron from the other atom. The atom that gained the electron is called an anion because it is negatively charged, while the atom lost the electron is called a cation and is positively charged. Because of their opposite charges, cations and anions are attracted to each other and form ionic bonds. An ion is a charged atom.
An example of an ionic bond is that between sodium and chlorine (Na+Cl-). A sodium atom has 11 electrons and 1 valence electron, while a chlorine atom has 17 electrons and 7 valence electrons. Because chlorine is much more electronegative than sodium, it will attract that one valence electron that sodium has. Thus, sodium has lost that valence shell completely and is complete, and chlorine has completed the valence shell that it has. Sodium becomes the cation while chlorine becomes the anion.
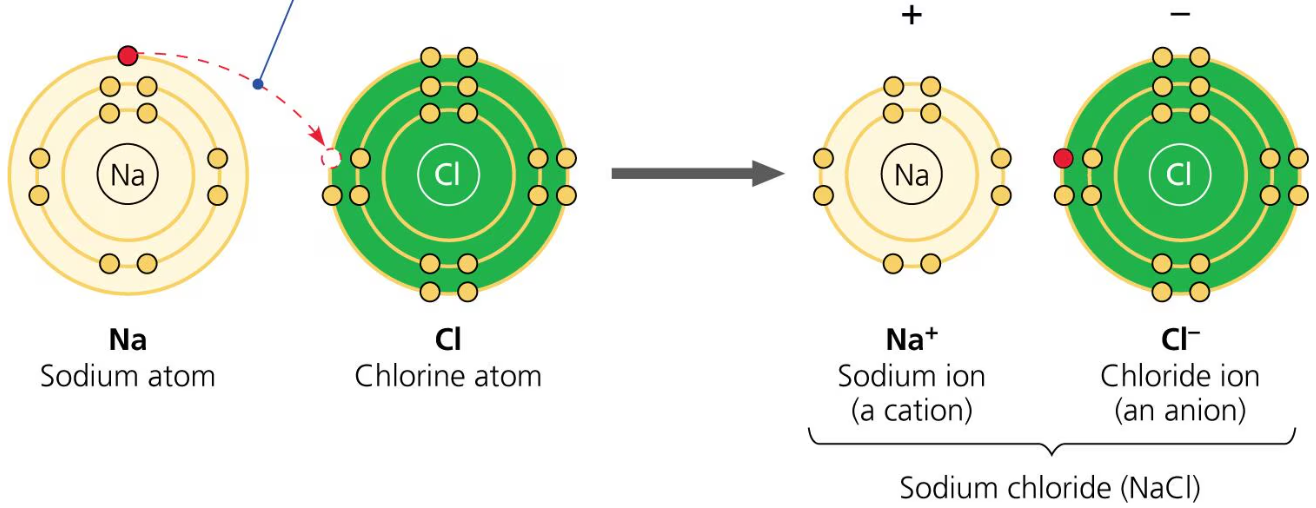
Compounds formed by ionic bonds are called ionic compounds or salts.
Unlike covalent compounds, ionic compounds do not consist of molecules. The formula for the ionic compound only indicates the ratio of elements in it. (ex. NaCl is not a molecule).
Not all ionic compounds have a 1:1 ratio of elements, magnesium chloride for example has two chloride ions for one magnesium ion.
Weak Chemical Interactions
In atoms, most strongest bonds are covalent bonds. However, weak bonds are also very important in organisms. One of these weak bonds are the ionic bonds which dissociate in water. Two other types of weak bonds are hydrogen bonds and van der Waals interactions.
Ionic compounds (such as NaCl) dissociate in water. This is because hydrogen is slightly positive and oxygen is slightly negative in water. These slight charges make Na+Cl- dissociate or dissolve.
NaCl dissolves in water because of this charge, however, other molecules such as (O2) will not dissolve as much in water because they aren’t charged. This property NaCl has of being able to dissolve in water is called solubility.
Hydrogen Bonds
Hydrogen bonds are weak bonds that are incredibly important in the world of chemistry.
When hydrogen (not electronegative) is covalently bonded to a very electronegative element (such as oxygen), the hydrogen gets a slightly positive charge. This allows it to bond to a different electronegative atom with a slightly negative charge. This non covalent attraction between a hydrogen atom and an electronegative atom is called a hydrogen bond.
In living cells these electronegative partners are usually oxygen or nitrogen atoms.
Hydrogen bonds are very important because they are what give water special properties.
Hydrogen bonds are actually why water in the solid for (ice) floats and is less dense than liquid water.
Van der Waals Interactions
Even a molecule with nonpolar covalent bonds can have regions with positive and negative charges. Electrons are not always evenly distributed and they can accumulate in one part of a molecule by chance. These van der Waals interactions are weak and only occur when an atom and a molecule are very close together.
However, when many of these interactions occur at once, they can be very powerful.
The van der Waals interactions are what allow a gecko to climb straight up a wall.
Molecular Shape and Function
A molecule has a specific shape and function.
A molecule that consists of two atoms is always linear, but molecules with more than two atoms can have complicated shapes.
These shapes are determined by the positions of the atoms orbitals and when atoms form covalent bonds, the orbitals on their valence shells are rearranged.
Molecular shape is crucial because it determines how biological molecules recognize and respond to one another.