Equilibrium (IB)
Equilibrium Reactions in Chemistry
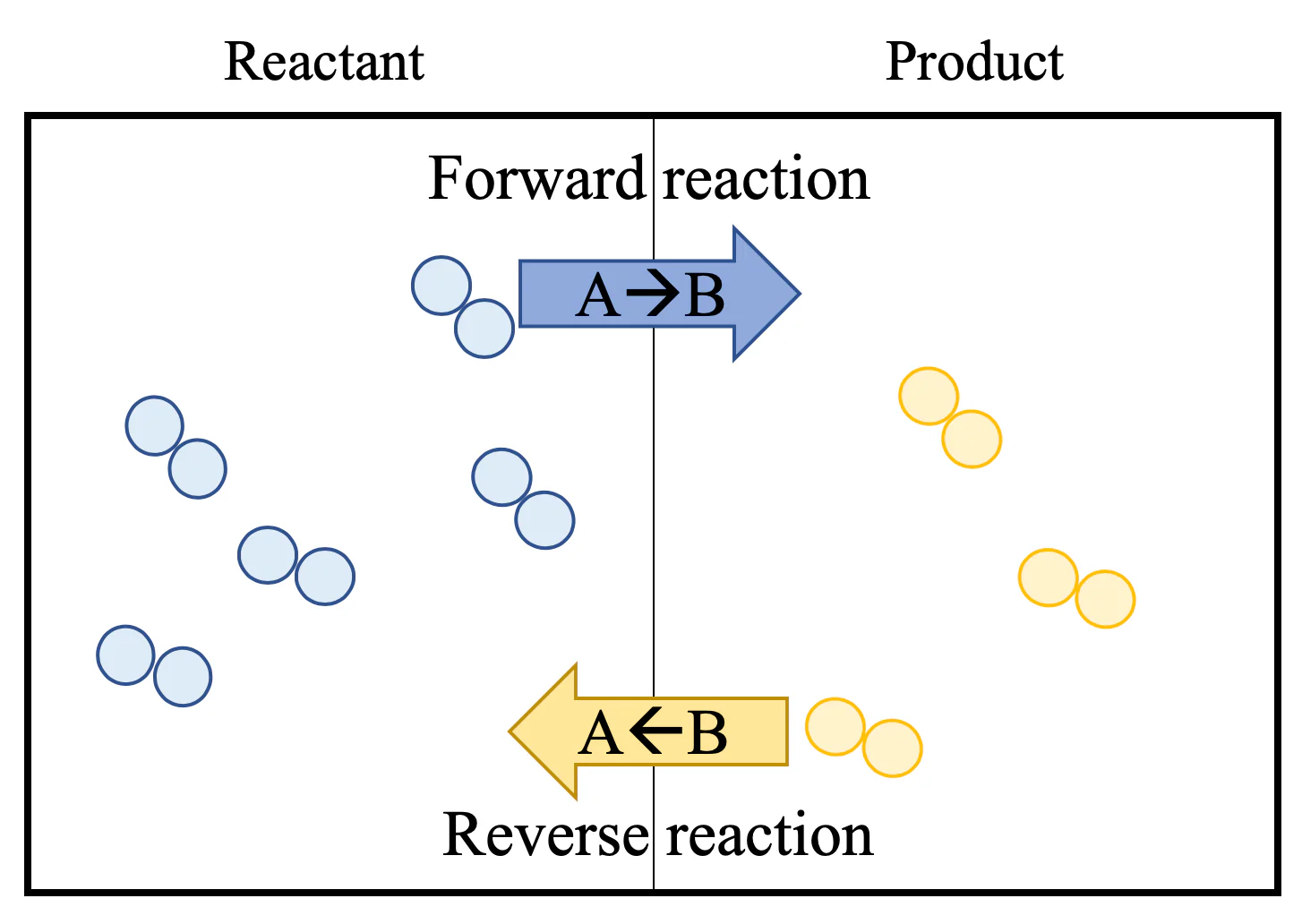
Many chemical reactions are reversible, meaning they can move in both directions: converting reactants into products (forward reaction) and converting products back into reactants (reverse reaction).
Reversible Reaction and Equilibrium:
In a reversible reaction, a state of dynamic equilibrium can be reached where:
The rates of the forward and reverse reactions are equal.
Reactants and products are continuously interconverted, but their overall concentrations remain unchanged over time.
This state of balance is reached in a closed system where no additional reactants or products are introduced or removed.
Example: Water in a Sealed Container:
When water in a sealed container is heated, some molecules in the liquid phase gain enough energy to escape into the gas phase (evaporation).
Since the container is sealed, some gas-phase molecules lose energy through collisions and return to the liquid phase (condensation).
Process to Equilibrium:
Initially: More liquid molecules evaporate than condense, as there is a high amount of liquid compared to vapor.
As Vapor Builds Up: The rate of condensation increases as more gas molecules are available to return to the liquid state.
At Equilibrium: The rates of evaporation and condensation are equal, resulting in no net change in the amounts of liquid and vapor. Molecules continue to transition between phases, but the total amounts of each phase remain constant.
Dynamic Equilibrium:
At equilibrium:
The forward and reverse reactions occur continuously at equal rates.
The concentrations of reactants and products stay constant, even though individual molecules keep interconverting.
Observable, or macroscopic, properties (such as color or density) remain unchanged, making the system appear static.
Indications of Equilibrium:
Single Arrow (→): Used for non-reversible reactions that proceed only in one direction.
Double Arrow (⇌): Used for reversible reactions where a dynamic equilibrium can be established.
Conditions for Chemical Equilibrium:
Closed System: To maintain equilibrium, reactants and products must be contained, allowing forward and reverse reactions to continue without interference. Examples include:
A gaseous reaction in a sealed container
A liquid, solid, or aqueous reaction in a closed beaker
Phase equilibrium (such as liquid-gas) in a sealed container
Constant Observable Properties: When a system is undisturbed at equilibrium, macroscopic properties (e.g., color, pressure, concentration) appear constant because opposing processes occur at equal rates.
Equal Forward and Reverse Rates: A reversible reaction at equilibrium has forward and reverse reaction rates that are balanced, ensuring that the concentration of reactants and products remains stable.
Temperature Control (Isothermal Systems):
Equilibrium systems are often maintained at a constant temperature, or isothermal conditions, by adding or removing heat as necessary to prevent changes in reaction rates due to temperature fluctuations.
Chemical Systems
Many reactions in chemistry reach a state called equilibrium, where both the forward and reverse reactions continue to occur.
The rates of these reactions depend on several factors, including:
Physical conditions: Temperature and pressure
Chemical conditions: Concentrations of reactants and products
Presence of a catalyst: Catalysts can speed up both the forward and reverse reactions but do not change the position of equilibrium.
Example: NO₂ and N₂O₄ Equilibrium
Nitrogen dioxide (NO₂) and dinitrogen tetroxide (N₂O₄) are gases that exist in a reversible equilibrium:
N₂O₄ (g)⇋2NO₂ (g)
N₂O₄ is a colorless gas, while NO₂ is a brown gas.
How Equilibrium Is Reached
Initially, the concentration of N₂O₄ is high, so its decomposition into NO₂ is rapid.
As NO₂ forms, some molecules collide and recombine into N₂O₄.
Over time, as N₂O₄ decreases and NO₂ increases, the rate of decomposition of N₂O₄ slows down, while the rate of NO₂ recombining into N₂O₄ speeds up.
Eventually, the forward reaction (N₂O₄ decomposing to NO₂) and the reverse reaction (NO₂ recombining into N₂O₄) occur at equal rates.
Reaching Dynamic Equilibrium
At dynamic equilibrium:
The rates of the forward and reverse reactions are equal.
There is no further change in the concentrations of N₂O₄ and NO₂.
Macroscopic properties, such as color and density, remain constant.
Characteristics of Dynamic Equilibrium
Both reactions continue to occur, but the overall concentrations of reactants and products do not change.
The equilibrium can be approached from either direction:
Starting with all N₂O₄, or all NO₂, the system will reach the same equilibrium concentrations.
If any conditions (temperature, pressure, concentration) change, the equilibrium position will shift.
Equilibrium Law
The law of chemical equilibrium states that at a given temperature, the ratio of the concentrations of products to reactants is constant when the system has reached equilibrium. This ratio is known as the equilibrium constant (denoted as Kₓ), and it varies depending on the temperature of the system.
General Form of the Equilibrium Constant Expression
For a reversible chemical reaction, such as:
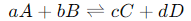
The equilibrium constant expression, KC, is written as:
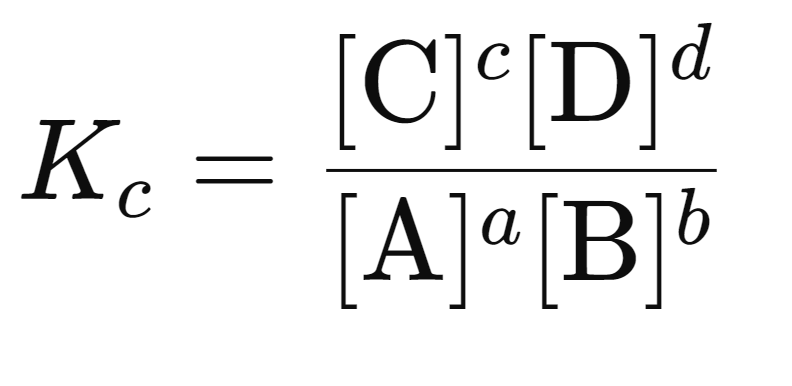
Where:
[A],[B],[C],[D] represent the concentrations of the reactants (A, B) and products (C, D), respectively.
The concentrations are raised to the power of their corresponding stoichiometric coefficients (a, b, c, and d) from the balanced equation.
Key Points About the Equilibrium Constant Expression
Numerator: The concentrations of products raised to the power of their coefficients.
Denominator: The concentrations of reactants raised to the power of their coefficients.
If there are multiple reactants or products, the terms are multiplied together.
Example: H₂(g) + I₂(g) ⇋ 2HI(g)
The equilibrium expression for this reaction is:
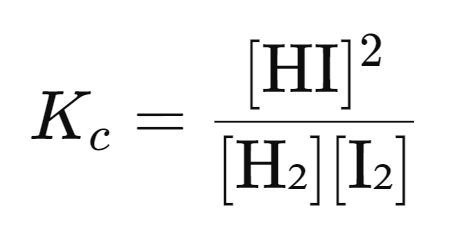
Inverse Reaction
For the reverse reaction, the equilibrium constant expression is inverted:
Original reaction: aA + bB ⇋ cC + dD
Reverse reaction: cC + dD ⇋ aA + bB
The equilibrium constant for the reverse reaction is:
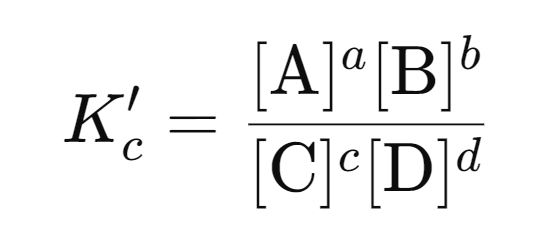
It is important to note that:
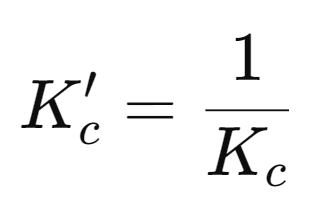
Reactions with Changed Stoichiometry
Doubling the Coefficients:
If the coefficients of the reaction are doubled, the new equilibrium constant expression will have the concentrations raised to twice the original powers. For example:
2aA+2bB⇋2cC+2dD
The equilibrium constant, KCx, will be: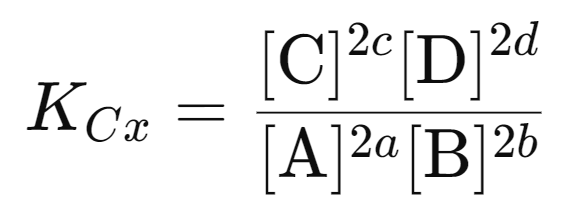
Halving the Coefficients:
If the coefficients are halved, the equilibrium constant expression will involve the square root of the original equilibrium constant Kc:
Summing Reactions:
If two or more reactions are combined, the equilibrium constant for the overall reaction is the product of the equilibrium constants of the individual reactions:
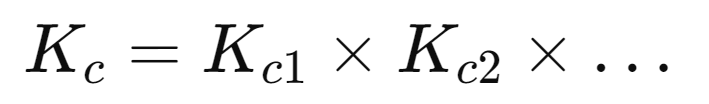
Types of Equilibria
Homogeneous Equilibrium: All reactants and products are in the same phase (usually gas phase). For example, reactions occurring entirely in the gas phase.
Heterogeneous Equilibrium: Reactants and products exist in more than one phase (solid, liquid, gas). For example, a reaction between a solid and a gas.
Important Considerations
Pure Solids and Liquids: In equilibrium expressions, pure solids and pure liquids are not included because their concentrations do not change and are considered constant.
Effect of Temperature: The equilibrium constant Kc changes with temperature. A higher Kc (greater than 1) suggests that products are favored at equilibrium, whereas a smaller Kc (less than 1) indicates that reactants are favored.
Vapor Pressure and Phase Equilibrium
Vapor Pressure is the pressure exerted by the gas phase of a substance when it is in equilibrium with its liquid phase.
In phase equilibrium (e.g., liquid ↔ gas), the system reaches a point where the rate of evaporation equals the rate of condensation.
The vapor pressure is a characteristic of the liquid and depends on temperature.
The size and shape of the container do not affect the equilibrium vapor pressure.
Effects of Conditions on Equilibrium Constant
When a system is at equilibrium, the position of equilibrium remains stable as long as temperature and pressure stay constant. However, certain experimental changes can affect the equilibrium position or shift the balance of reactants and products. While the equilibrium position may shift in response to changes, the equilibrium constant Kc itself only changes with temperature.
Summary of Changes in Conditions on Equilibrium
Condition | Equilibrium Position | Kc Value |
|---|---|---|
Concentration | Changes position (shifts to counterbalance) | No change |
Pressure | Shifts in gaseous reactions | No change |
Temperature | Shifts depending on reaction type | Changes |
Catalyst | No effect on position | No change |
1. Effect of Concentration Changes
Adding or removing reactants or products will shift the equilibrium position to counter the change. This is in line with Le Chatelier’s Principle, which states that a system at equilibrium will adjust to counteract an external change.
Adding reactants shifts equilibrium towards the products (forward reaction) to consume the added substance.
Removing reactants shifts equilibrium towards the reactants (reverse reaction).
Equilibrium constant Kc remains unchanged because it is only influenced by temperature.
2. Effect of Pressure Changes (for Gaseous Reactions)
In reactions involving gaseous reactants and products, changes in pressure can impact the equilibrium position.
Increasing pressure will shift the equilibrium toward the side with fewer gas molecules, which helps reduce overall pressure in the system.
For example, in a reaction where 4 moles of gaseous reactants produce 3 moles of gaseous products, an increase in pressure will favor the forward reaction to reduce the number of moles.
Decreasing pressure will favor the side with more gas molecules.
Kc remains constant because pressure does not directly alter the equilibrium constant; it only shifts the position.
3. Effect of Temperature Changes
Temperature is unique in that it directly affects both the equilibrium position and the value of Kc:
Exothermic Reactions:
Heat is a product in exothermic reactions. Adding heat (increasing temperature) causes the equilibrium to shift left, favoring the reactants, to reduce the added heat.
Decreasing temperature shifts equilibrium right, favoring the products.
Kc changes based on the temperature change:
For exothermic reactions, increasing temperature decreases Kc as the system favors reactants.
For endothermic reactions, increasing temperature increases Kc as the system favors products.
Endothermic Reactions:
Heat is a reactant in endothermic reactions. Adding heat shifts equilibrium to the right to absorb the added energy.
Kc increases with temperature for endothermic reactions because the products are favored.
4. Effect of a Catalyst
A catalyst lowers the activation energy of both the forward and reverse reactions, speeding up the time it takes to reach equilibrium but not changing the equilibrium position or Kc.
It does not change the equilibrium constant or shift the balance of products and reactants.
Summary of Temperature Effects on Equilibrium Direction
Reaction Type | Increase in Temperature | Decrease in Temperature |
|---|---|---|
Exothermic | Shifts left (reactants) | Shifts right (products) |
Endothermic | Shifts right (products) | Shifts left (reactants) |
Note: In any reaction where the enthalpy change ΔH=0, temperature changes have no effect on Kc since no heat is absorbed or released.
Le Chatelier’s Principle
Le Châtelier’s Principle explains how a system at equilibrium responds to changes in conditions (concentration, temperature, or pressure) to maintain or restore balance. When an external change disturbs a system in equilibrium, the system will adjust itself to offset the change and re-establish equilibrium. This principle helps predict the direction in which equilibrium shifts when the system is altered.
Key Influencing Factors on Equilibrium
Concentration Changes
When the concentration of a reactant increases or a product decreases, the system is no longer at equilibrium. To offset this, the forward reaction is favored to convert some of the added reactants into products, moving the system back toward equilibrium.
When the concentration of a product increases or a reactant decreases, the reverse reaction is favored, shifting equilibrium toward the reactants to counteract the imbalance.
Kc (equilibrium constant) remains unchanged by concentration changes, as only temperature affects its value.
Temperature Changes
The direction of the equilibrium shift due to temperature depends on whether the reaction is endothermic (absorbs heat) or exothermic (releases heat).
Endothermic Reactions (ΔH>0\Delta H > 0ΔH>0):
Increasing temperature adds heat, which acts like a reactant in endothermic reactions, so the forward reaction is favored, and equilibrium shifts toward the products.
Decreasing temperature removes heat, favoring the reverse reaction, shifting equilibrium toward the reactants.
Exothermic Reactions (ΔH<0):
Increasing temperature adds heat, acting as an excess product, so the system responds by favoring the reverse reaction and shifting equilibrium toward the reactants.
Decreasing temperature removes heat, favoring the forward reaction and shifting equilibrium toward the products.
Effect on Kc: Unlike other factors, temperature changes the equilibrium constant (Kc) because heat is either absorbed or released, impacting the system’s balance.
Pressure Changes (for Reactions with Gaseous Species)
For reactions involving gases, pressure changes affect equilibrium if there is a difference in the number of gas molecules (moles) between the reactants and products.
If products have fewer gas molecules:
Increasing pressure favors the side with fewer gas molecules to reduce pressure, shifting equilibrium toward the products.
Decreasing pressure favors the side with more gas molecules, shifting equilibrium toward the reactants.
If reactants have fewer gas molecules:
Increasing pressure shifts equilibrium toward the reactants, favoring the reverse reaction.
Decreasing pressure shifts equilibrium toward the products, favoring the forward reaction.
Effect on Kc: Pressure changes do not alter Kc, as it is only dependent on temperature.
Effect of a Catalyst
A catalyst speeds up both the forward and reverse reactions equally by lowering their activation energy, allowing the system to reach equilibrium more quickly.
Equilibrium Position: The catalyst does not shift the equilibrium position since it affects both reactions equally.
Equilibrium Constant Kc: Adding a catalyst does not change Kc because it does not affect the relative concentrations of reactants and products at equilibrium.
Summary of How Le Châtelier’s Principle Affects Equilibrium Shifts
Condition Change | Effect on Equilibrium Position | Effect on Kc |
|---|---|---|
Increase in Reactant | Forward reaction favored, shifts to products | No change |
Increase in Product | Reverse reaction favored, shifts to reactants | No change |
Increase in Temperature (Endothermic) | Forward reaction favored, shifts to products | Increases Kc |
Decrease in Temperature (Endothermic) | Reverse reaction favored, shifts to reactants | Decreases Kc |
Increase in Temperature (Exothermic) | Reverse reaction favored, shifts to reactants | Decreases Kc |
Decrease in Temperature (Exothermic) | Forward reaction favored, shifts to products | Increases Kc |
Increase in Pressure (fewer gas moles in products) | Shifts to products | No change |
Increase in Pressure (fewer gas moles in reactants) | Shifts to reactants | No change |
Catalyst Addition | No effect on position, equilibrium reached faster | No change |
Le Châtelier’s Principle helps predict how a system in equilibrium will respond to various changes, guiding the direction of reaction shifts to maintain or re-establish equilibrium.
Reaction Quotient
The concept of the reaction quotient (denoted by Q) and how it compares to the equilibrium constant (Kc) is essential for understanding the progress of a chemical reaction as it moves toward equilibrium. Let me explain it step by step to ensure clarity.
Reaction Quotient (Q)
The reaction quotient Q is a mathematical expression similar to the equilibrium constant Kc, but the key difference is that Q is calculated using the concentrations of reactants and products at any point in time, whether or not the system is at equilibrium.
For a generic reaction of the form:
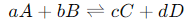
The reaction quotient Q is given by:
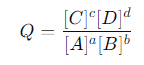
where [C],[D],[A], and [B] are the molar concentrations of the chemical species C, D, A, and B at a given moment in time. This expression is exactly the same as the equilibrium expression for Kc, but instead of equilibrium concentrations, Q uses the concentrations at any moment.
Comparing Q and Kc
When Q=Kc:
The reaction is at equilibrium.
At equilibrium, the forward and reverse reactions occur at the same rate, so the concentrations of reactants and products do not change. The system is balanced, and no net change in the concentrations will occur unless the conditions (e.g., temperature or pressure) change.
When Q>Kc:
This means that, at this point in time, the concentration of products is higher than it would be at equilibrium.
Since the reaction tends to favor the direction that reduces the concentration of products and increases the concentration of reactants to reach equilibrium, the reverse reaction will be favored.
The system will shift toward the left (toward the reactants) to decrease the concentration of products and increase the concentration of reactants, moving toward equilibrium.
When Q<Kc:
This indicates that the concentration of reactants is higher than it would be at equilibrium.
To reach equilibrium, the system will favor the forward reaction (the production of products).
The reaction will shift to the right (toward the products) to decrease the concentration of reactants and increase the concentration of products, moving toward equilibrium.
How Q Helps Predict the Reaction's Direction
The value of Q gives you a snapshot of the system's current state relative to equilibrium. By comparing Q to Kc, you can predict:
If Q<Kc, the reaction will move forward (toward the products) to reach equilibrium. The system needs to produce more products and consume more reactants.
If Q>Kc, the reaction will move backward (toward the reactants) to reach equilibrium. The system needs to decrease the amount of products and generate more reactants.
If Q=Kc, the system is already at equilibrium, and no net change will occur in the concentrations of reactants and products.
Significance of the Reaction Quotient
Helps predict the shift: By knowing the value of Q compared to Kc, you can determine whether the reaction needs to proceed forward (to make more products) or backward (to make more reactants) to achieve equilibrium.
Indicates the direction of change: The reaction will always move in the direction that brings the system closer to equilibrium. If there are too many products (when Q>Kc), the system will shift toward reactants. If there are too many reactants (when Q<Kc), the system will shift toward products.
Example
Let's take an example to illustrate how Q works.
Consider the following reaction:
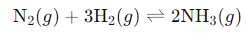
The equilibrium expression for this reaction is:
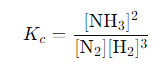
Now, suppose at a given time the concentrations are as follows:
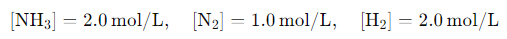
The reaction quotient at this point would be:
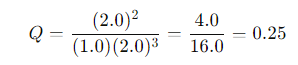
If the equilibrium constant Kc = 1.0, we see that:
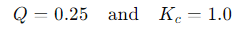
Since Q<Kc, the concentration of reactants (N2 and H2) is greater than at equilibrium, and the system will shift forward to produce more NH3, moving toward equilibrium.