Chapter 3: Speaking with Pictures: Drawing Structures
Organic Chemistry for Dummies 2nd Edition by Arthur Winter, PhD
Chapter 3: Speaking with Pictures: Drawing Structures
==Picture-Talk: Lewis Structures==
- Lewis structure: shows what atoms are connected to each other, and it shows where the electrons in the molecule reside.
- Single bonds between two atoms are represented with a single line, signifying two shared electrons.
- Double bonds are represented with a double line, signifying four shared electrons.
- Triple bonds are represented with a triple line, signifying six shared electrons.
- Nonbonding electrons are indicated with dots on the atoms on which they reside.
@@Taking charge: Assigning formal charges@@
How to determine the formal charge on an atom?
- %%Equation: formal charge on an atom = valence electrons - dots - sticks.%%
- @@Dots:@@ number of lone-pair electrons around the atom.
- @@Sticks:@@ number of bonds off the atom.
- A single bond is one stick; a double bond is two; a triple bond is three).
- ==For example==: for NH2, the formal charge equation for nitrogen in this molecule is:
- formal charge = 5 valence electrons – 4 dots – 2 sticks = –1
The formal charge on nitrogen, then, is -1, so nitrogen is anionic in this molecule.
This can be done for each of the atoms in this molecule.
Valency: number of bonds around a neutral atom.
- ==Examples==:
- Carbon is tetravalent as it has 4 bonds to other atoms when it’s neutral.
- Nitrogen is trivalent because it has 3 bonds to other atoms (plus a lone pair) when it’s neutral
@@Drawing Structures@@
- A Lewis structure explicitly draws out all the bonds in a molecule, but this can be tedious so there is a shorthand notation.
@@Atom packing: Condensed structures:@@
Condensed structure: structure abbreviation of the full Lewis structure. The bonds between carbon and hydrogen are not explicitly shown but instead each carbon and the attached hydrogens are grouped together into a cluster (such as CH2 or CH3).
- ==Example with diethyl ether:==
Most useful when the molecule consists of strain chains of atoms as structures that contain rings are difficult to portray with this structure.
Parentheses with subscripts are used to abbreviate when two or more identical groups are attached to an atom. They can also be used when a chain becomes very long and a unit is repeated many times.
@@Structural shorthand: Line-bond structures@@
Line-bond structure: most common method of structure drawing.
- Rules for line-bond structures:
- Each point on a jagged line is assumed to be a carbon atom.
- The ends (tips) of lines are assumed to be carbon atoms.
- All hydrogens attached to non-carbon elements (like N, O, S, and so on) must be explicitly shown.
- All atoms are presumed to be neutral unless a charge is specifically shown.
- ==Example shown with isooctane:==
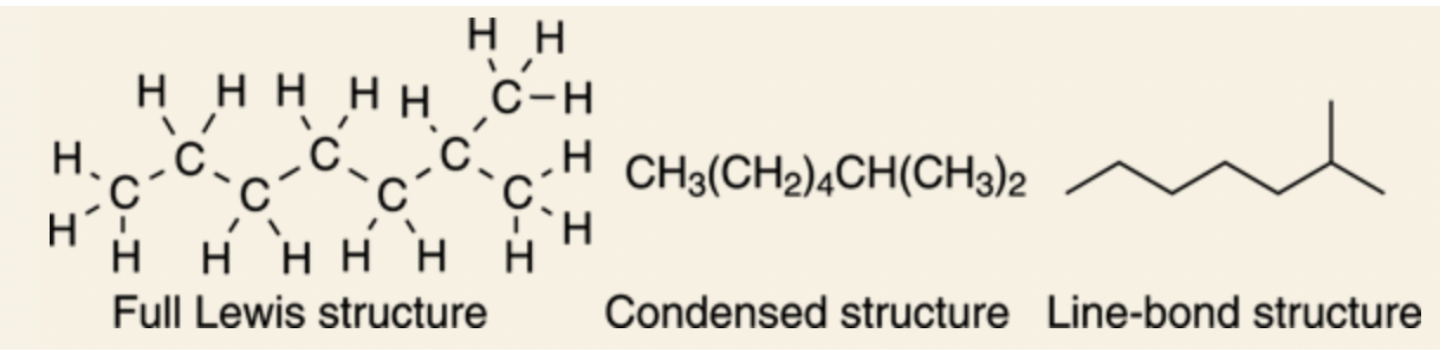
@@Converting Lewis structures to line-bond structures@@
Jagged line: represents straight chain molecules, with each point (including the ends of the structure) representing a carbon atom.
- ==Example shown with hexane:==
Rings are represented by polygons, with each point representing a carbon atom.
- A triangle is a three-carbon ring (which, of course, is the smallest possible ring size).
- A square is a four-carbon ring.
- A pentagon is a five-carbon ring; and so forth.
- ==Example shown with cyclopentane==
Multiple bonds are explicitly drawn out. Triple bonds are drawn in a straight line.
- ==Example shown with diisopropyl acetylene.==
Hydrogens attached to any atom other than carbon (atoms such as oxygen [O], sulfur [S], and nitrogen [N]) must be explicitly shown; only hydrogens attached to carbons are not shown.
- ==Example shown with ethanolamine:==
\n
@@Determining the number of hydrogens on line-bond structures@@
A neutral carbon has four bonds.
A line-bond structure showing a carbon that has three bonds to other atoms, it must have one implicit hydrogen.
If it shows a carbon that has two bonds, it must have two implicit hydrogens.
If it shows a carbon that has only one bond, it must have three implicit hydrogens.
- ==Example shown:==
@@So lonely: Determining lone pairs on atoms@@
- Plug the information into the rearranged equation for the formal charge, where dots is simply the number of nonbonding electrons.
- dots = valence electrons – sticks – formal charge
- ==Example with NH2:==
- Plug into the equation the number of valence electrons for nitrogen (5), the number of sticks (2, because nitrogen has two bonds, one with each of the two hydrogens), and the formal charge on the atom (–1 in this case)
- dots = 5 – 2 – (–1) = 4
- NH2– has four nonbonding electrons, or two lone pairs of electrons (because there are two “dots” in every lone pair).
==Problem Solving: Arrow Pushing==
Resonance arrow: Used to indicate movement between resonance structures.
Equilibrium arrow: Used to show reactions governed by equilibria. Making one of the equilibrium arrows longer shows the direction of the equilibrium in a reaction (in other words, which side of the reaction — reactants or products — is favored).
Reaction arrow: Used to show the change of molecules by a reaction.
Full-headed arrow: Used to show the movement of two electrons; involve the movement of lone pairs and bonds — each of which contains two electrons.
- Note: Draw arrows from electrons toward where they’re going, never the other way around.
Half-headed arrow: Used to show the movement of one electron; used for describing free radical reactions because these reactions involve the movement of single electrons.
==Example using water:==
- If, for example, you want to show water being protonated (receiving an H+ ion) by acid, you would show one of the lone pairs on water’s oxygen attacking the proton (the H+), not the proton moving onto the water.
==Drawing Resonance Structures==
@@Resonance structures:@@ used to to account for a flaw in showing the locations of certain lone-pair and pi electrons in Lewis structures (pi electrons are electrons found in pi bonds.
- ==Example with carboxylate anion:==
- This Lewis structure predicts one double bond between carbon and one of the oxygens, and one single bond between carbon and the oxygen that contains the negative charge, so that the two C-O bonds are different.
- The actual structure is one in which both of the carbon-oxygen bonds are identical — with each C-O bond having some single-bond qualities and some double-bond qualities — and each of the oxygens shares the negative charge equally.
Any time more than one valid Lewis structure can be drawn for a given molecule, each of these alternate structures is considered a resonance structure.
- The actual structure of a molecule will look like a hybrid of all the different resonance structures.
@@Rules for resonance structures@@
Atoms are fixed and cannot move. Only the distribution of the electrons in the molecule changes from one resonance structure to the next.
Only lone-pair electrons and pi electrons (only found in double and triple bonds) can move. Single bonds cannot move at all.
You cannot break the octet rule. The sum of an atom’s lone pairs and bonds cannot be greater than 4 for the second-row elements.
Individual Lewis structures in a resonance structure do not properly represent the actual molecule.
- In the case of the carboxylate anion, it has two resonance structures.
- Neither one resonance structure is the correct one. The correct structure is a hybrid of both of the resonance structures.
- In the case of the carboxylate anion, each of the C-O bonds is neither a double bond nor a single bond, but a bond that is somewhere in between (one-and-a-half bonds).
The double-headed resonance arrow shows that these resonance structures are equivalent, and a way of correcting the flaw in Lewis theory and accounting for the proper electron distribution.
@@Problem solving: Drawing resonance structures@@
Pushing around the non-bonding and pi electrons with arrows.
- Electron-pushing arrows are used to convert one resonance structure into the next.
Resonance structures use arrows to determine all the forms of resonance structures rather than the movement of electrons.
Four structural features of molecules with 2 or more resonance structures
- ^^A lone pair next to a double bond or triple bond^^
- Arrows can be used to convert one resonance structure into the other.
In cases where a lone pair is adjacent to a double bond, at least two arrows are drawn.
- The first begins at the lone-pair electrons and moves these electrons in the direction of the double bond.
- The next arrow places the double bond electrons onto the adjacent carbon as a lone pair.
- Note: If you had stopped right after drawing the first arrow, it would have violated the octet rule.
^^A cation next to a double bond, triple bond, or lone-pair of electrons^^
In this case, you can’t draw the arrow from the positive charge because there are no lone-pair or pi electrons on that carbon.
For double/triple bonds:
- Instead, move the double-bond electrons onto the adjacent single bond, making a new double bond.
- This re-forms the double bond on the other side of the molecule and shifts the positive charge from the left side to the right.
For lone pairs:
- Move the lone pair of electrons in the direction of the cation to form a double bond.
The positive charge then moves to the atom that originally held the lone pair, because that atom has become deficient in electrons.
^^A double or triple bond containing an electronegative atom^^
- Move one of the double (or triple) bonds onto the electronegative element as a lone pair.
- In such situations, the electrons always go onto the more electronegative element, not the carbon.
- Example shown with acetone:
Alternating double bonds around a ring:
- Move each of the double bonds over to the next carbon to make an alternative resonance form.
- Example shown with benzene:
@@Drawing more than two resonance structures@@
- ==Example of 2-hexadienone==, which has four resonance structures:
Recognize it has a double bond containing an electronegative atom, which conforms to pattern number three described in the preceding section.
Draw the resonance structure by moving the double-bond electrons onto the oxygen to create a species with a positive charge on carbon.
The positive charge, now, is next to a double bond, which is another of the common patterns (pattern number two mentioned previously). Another resonance structure can be drawn by moving the double-bond electrons onto the single bond to the left, which moves the location of the positive charge.
Move the last double bond over to the left in the same fashion as you did previously and put the positive charge on the end carbon atom.
@@Assigning importance to resonance structures@@
More stable resonance structures will contribute more to the hybrid than unstable ones.
Three major factors to determine relative stability of the resonance structures.
^^Fewest charges^^
Keeping positive and negative charges away from each other costs energy for the molecule, so it is more stable to have the fewest charges.
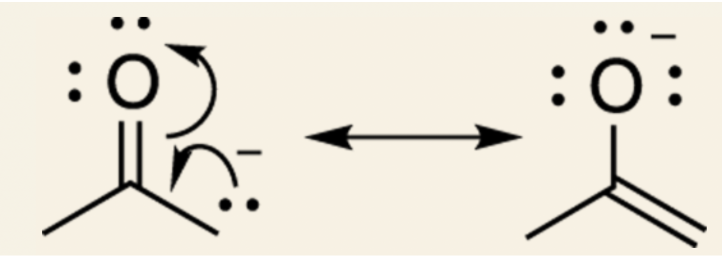
Example with acetone: the first resonance structure is uncharged so it contributes more to the overall hybrid.
^^Charges on the best atoms^^
Negative charges generally prefer to rest on electronegative elements (elements such as oxygen and nitrogen), while positive charges will prefer to rest on electropositive elements (such as carbon).
Example with acetone: the negative charge rests on the carbon and the positive charge rests on the oxygen.
- This resonance structure is considered a bad resonance structure. Because oxygen is an electronegative atom (an electron pig), it really does not want to have a positive charge; therefore, the resonance structure on the right would contribute an insignificant amount to the overall hybrid.
Whereas, here both resonance structures would contribute to the overall hybrid.
- Because the signs are on the appropriate atoms of the molecule.
^^Filled Octets^^
- Every atom of the molecule should try to have a full octet of electrons.
- Example shown with CN^-:
- The resonance structure on the right contributes the most to the overall hybrid, because every atom in that resonance form has a full octet of electrons.
- In the resonance structure on the left, the carbon does not have a complete octet, owning only six electrons in its outermost shell.
@@Common mistakes in drawing resonance structures@@
- ^^Forgetting charges^^
- To quickly check for missing charges is to see if all your charges balance.
- Check if the net charge that you begin with equals the net charge that you end up with.
- If your starting resonance structure has a net negative charge, then all other resonance structures must have a net negative charge as well.
- ^^Breaking the octet rule^^
- Remember that an atom in the second row of the periodic table (like C, N, O, or F) will never have more than eight electrons around it.
- This means that the sum of all the bonds and lone pairs around one of these atoms cannot total more than four.
- ^^Moving single bonds^^
- Remember only lone-pairs and pi electrons (electrons in double or triple bonds) can move.
- ^^Not following the electron flow^^
- Make sure they are drawn “flowing” in a single direction, not starting one way and doubling back in the opposite direction.