Ap Biology (copy)
Unit 1: The Chemistry of Life/ Mini unit: statistics
The Chemistry of Life ( main unit )
Atoms: functions and structure
Matter is anything that takes up space and has mass; it is composed of atoms, which are the basic units of matter.
A chemical substance is a material which has a definite chemical
composition
chemical substance can be a sole element or a chemical compound
Elements are pure substances that CANNOT be broken down into different types of substances( think: gold, carbon, sulfur)
Each one of the examples is only made up of 1 type of atom
Atoms are the smallest particles of an element
The center of an atom is the “nucleus”
the nucleus holds positively and neutrally charged particles called “protons” and '“neutrons”
Around the atom their a cloud called an “Electron cloud” which is made up of negatively charged electrons. These electrons are arranged by energy level
Atoms are electrically neutral if there is an equal amount of protons as their electrons
If an atom gains or loses electrons, it becomes an ion, carrying a positive or negative charge.
Because these “subatomic particles” are so small we have to use a different system of weight called a “Dalton”(Da).
neutrons and protons have almost the same mass, which is about 1 Dalton, while electrons have a negligible mass compared to them, approximately 1/2000 of a proton's mass.
The atomic number is defined as the number of protons found in the nucleus of an atom, which determines the element's identity. it is written and subscript to the left bottom(for example helium: ²He
the 2 tells us that there are 2 protons in its nucleus
The atomic number also tells us the number of electrons in the atom( as long as the atom is neutral and doesn’t state it as an ion)
To try and get the number of neutrons we use “mass number”
The mass number is the sum of protons plus neutrons in the nucleus
it is written as superscript to the left of the element symbol (example: helium 42He where the 4 means the total amount of protons and neutrons, the 2 means the protons, we can subtract 4 by 2 and see that there are 2 neutrons in helium)
The atomic weight of an atom is the weight average of the protons and neutrons of an atom
Since both protons and neutrons weigh the same(about 1 Dalton) we can just combine their weights (He had an atomic weight of about 4 because it had 2 neutrons and 2 protons)
A Chemical compound is a “new” substance made when 2 or more atoms of an element react together
A chemical reaction is a process which changes some chemical substance into other chemical substances
Any chemical compound which results from a reaction always has a unique and fixed chemical composition
The only to separate the substances in a compound is by another chemical
reaction
Isotopes occur when There are more neutrons than protons in an atom
-(their are 3 main carbons, Carbon-12, Carbon-13, and Carbon-14, each has their own amount of the neutrons and protons. carb-12 has 6 protons with 6 neutrons, carb 13 has 6 protons with 7 neutrons, and carb 14 has 6 protons with 8 neutrons. yet carb-14 is radioactive because of its instability
A radioactive isotope happens when the nucleus of an atom decays quickly giving off particles and energy
Atoms are mostly empty space
When a chemical reaction happens, it’s mostly the electrons that come into contact
Energy is the ability to do work
Potential energy is energy that matters stored b/c of location/position
(think of a ball on a hill, because its on the top of a hill when its going down its using that energy until it stops at the bottom)
Electrons also have potential energy, because of their position compared to the nucleus, the further they are from the nucleus the more potential energy they have
There are different states of potential energy that electrons have in an atom, which are called “energy levels” or “electron shells”
1st shell lowest. 2nd shell 2nd lowest, they keep increasing in energy the more shells are added
An electron can change its shell, the only way is to absorb or lose an amount of energy equal to the difference in potential energy between the old and new shell
To move an electron shell, it has to gain/absorb more energy(it goes further out) or lose energy by releasing it to the environment [usually in the form of heat]( it gets closer to the nucleus)
The chem behavior of an atom is determined by the configuration which is how the electrons of the electron shell are laid out
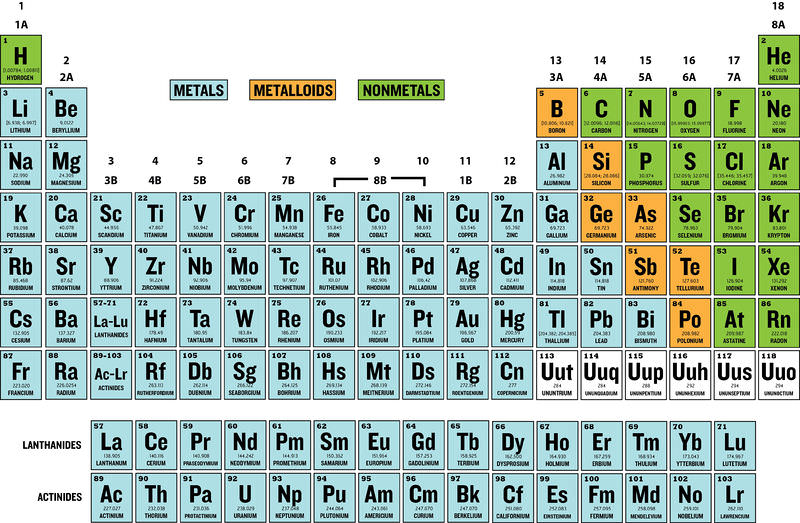
to know how many electrons can fit in a shell we must know which shell is it, the formula to find how many can fit is: 2(n)² where n = # of the shell (for example, hydrogen has one shell, 2(1)² = 2, it can only fit 2 electrons in the atom)
The chemical behavior of an atom mostly depends on the # of the outermost shell
The outermost electrons are called “valence electrons”
The outermost shell is called the “ Valence shell”
Atoms with the same # of electrons in their valence shells given similar chem behavior
such as fluorine and chlorine, they both have 7 valence electrons and are combined with sodium
Atoms with full valence shells are unreactive, meaning they won’t interact easily with other atoms that they come across( these atoms are said to be “inert” which means chemically unreactive)
Atoms that have incomplete shells interact with their partner atom in such a way that they complete their valence shell, they do this by sharing or transferring the valence electrons
These interactions cause atoms to stay close together and are held by attractions called “chemical bonds”
the strongest kinds are covalent and ionic
covalent bonds are the sharing of a pair of valence electrons by two atoms
let’s take hydrogen, it has 1 valence electron in its first and only only shell, but the capacity of the shell is two. when hydrogen atoms come close, each hydrogen will now have 2 electrons
when 2 or more atoms are held together by covalent bonds it can be considered a molecole
in this case,e we have made a hydrogen molecule and it can be written as H-H in which the line means covalent bonds
this type of notation is called “structural formula” Another way can say this is like “H2” where the number is a subscript and tells us that there is 2 covalently bonded hydrogen atoms( this is called “molecular formula
double covalent bonds are the sharing of 2 pairs of valence electrons
let’s take oxygen, into total is has 6 valence electrons,and when they come together they share 4 electrons to complete both of the shells
Valence is the bonding capacity, which is equal to the number of unpaired electrons in the valence shell
oxygen has a valence of 2 because in the valence shell ,it can bond 2 more electrons
Electronegativity is the an atoms tendency to attract electrons which are shared in bonds/ or strip away an atom valence electrons
The more electronegative an atom is the stronger it pulls shared electrons to itself
Atoms of the same element are equally electronegative and share the bonds equally are called nonpolar covalent bonds(ex: H2 the covalent bonds are nonpolar because their electrons are being shared equally)

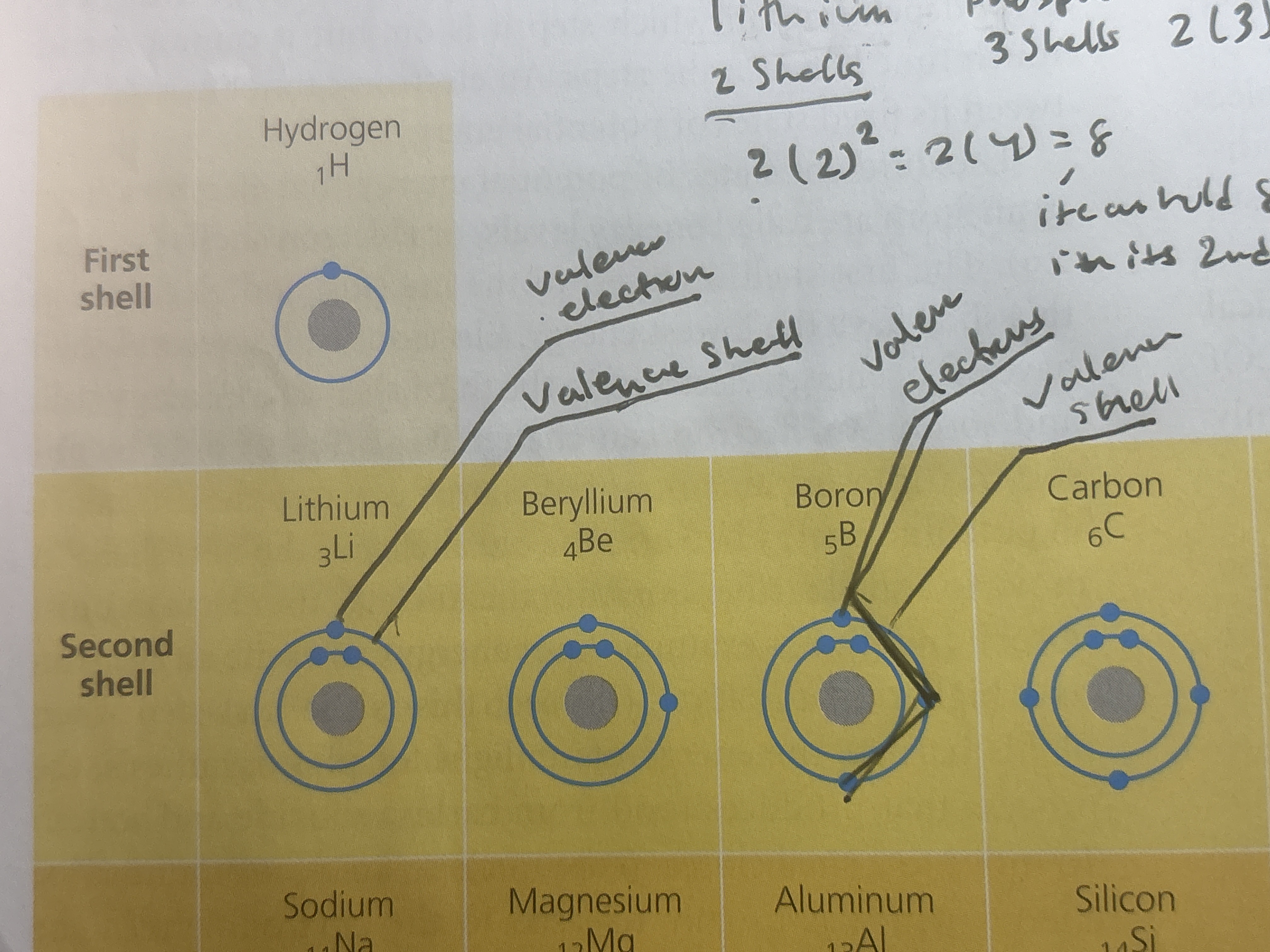
In polar covalent bonds when an atom is bonded to a more electronegative atom, the electrons will not be shared equally(ex: in a water molecule, since oxygen, is very electronegative the electrons of the hydrogen stay around the nucleus of the oxygen more often, and because of unequal sharing it causes a partial negative charge of the oxygen and the hydrogen to have a partial positive charge)
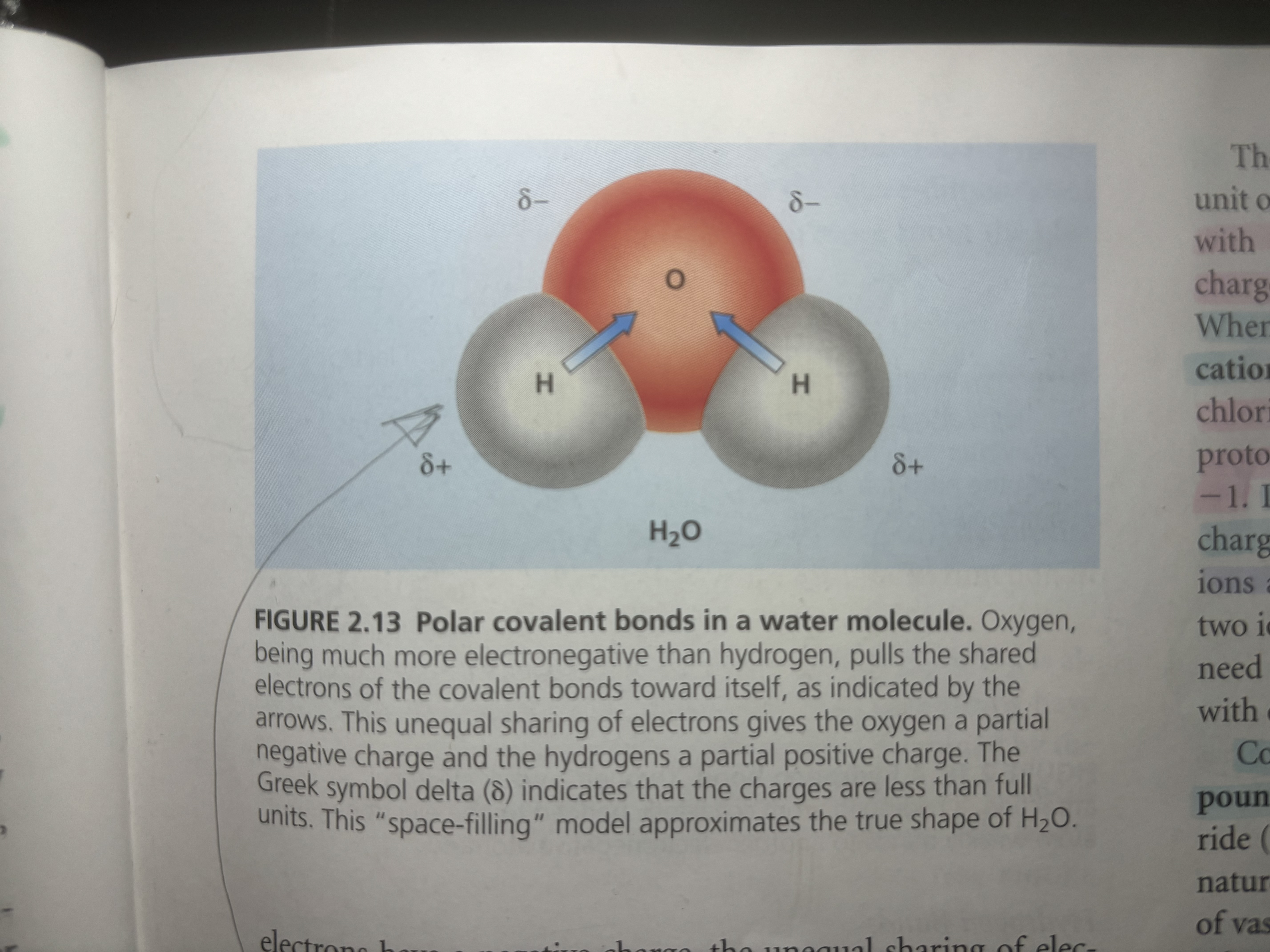
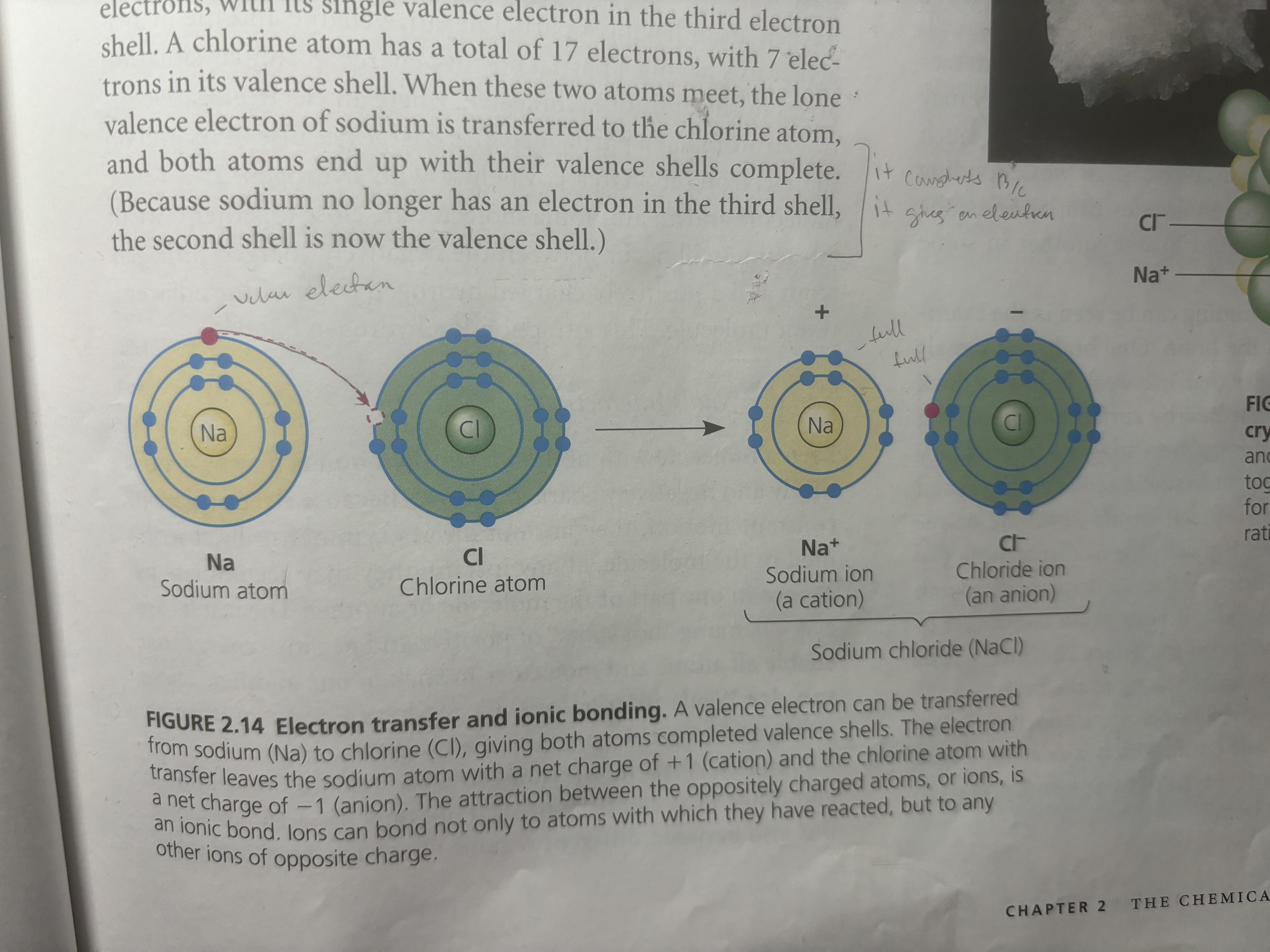
Ionic bonds are bonds in which one atom is very electronegative that it strips away the other atoms’ valence electron/and because of the opposite charges they attach other each (ex: salt when Na comes across Cl the Cl strips away the only valence electron that the Na has in the 3rd shell. Now Na has gained a + 1 positive charge [11 protons and 10 electrons] and Cl has gained an electron it has a negative charge of -1 [17 protons and 18 electrons])
An ion is when
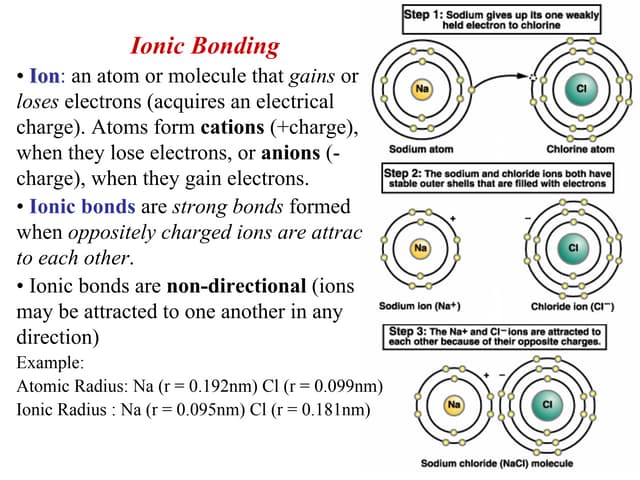
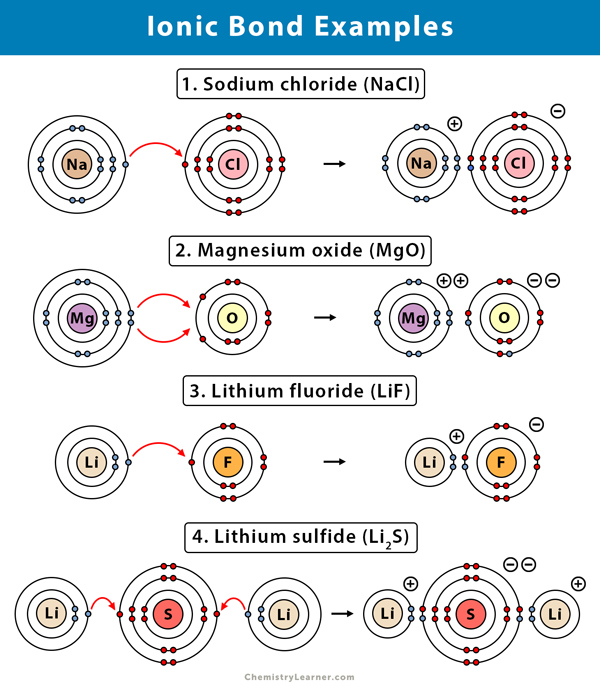
An ion is when an atom has gained an electron or proton
When it has gained a proton is it called a cation
When it has gained an electron, it is called an anion
Compounds made by ionic bonds are called ionic compounds or salts
Weak chemical bonds are forces of attraction between atoms/molecules which are pretty easy to break
Hydrogen bonds are bonds formed when a hydrogen atom is covalently bonded to one electronegative atom and is also attracted to another electronegative atom
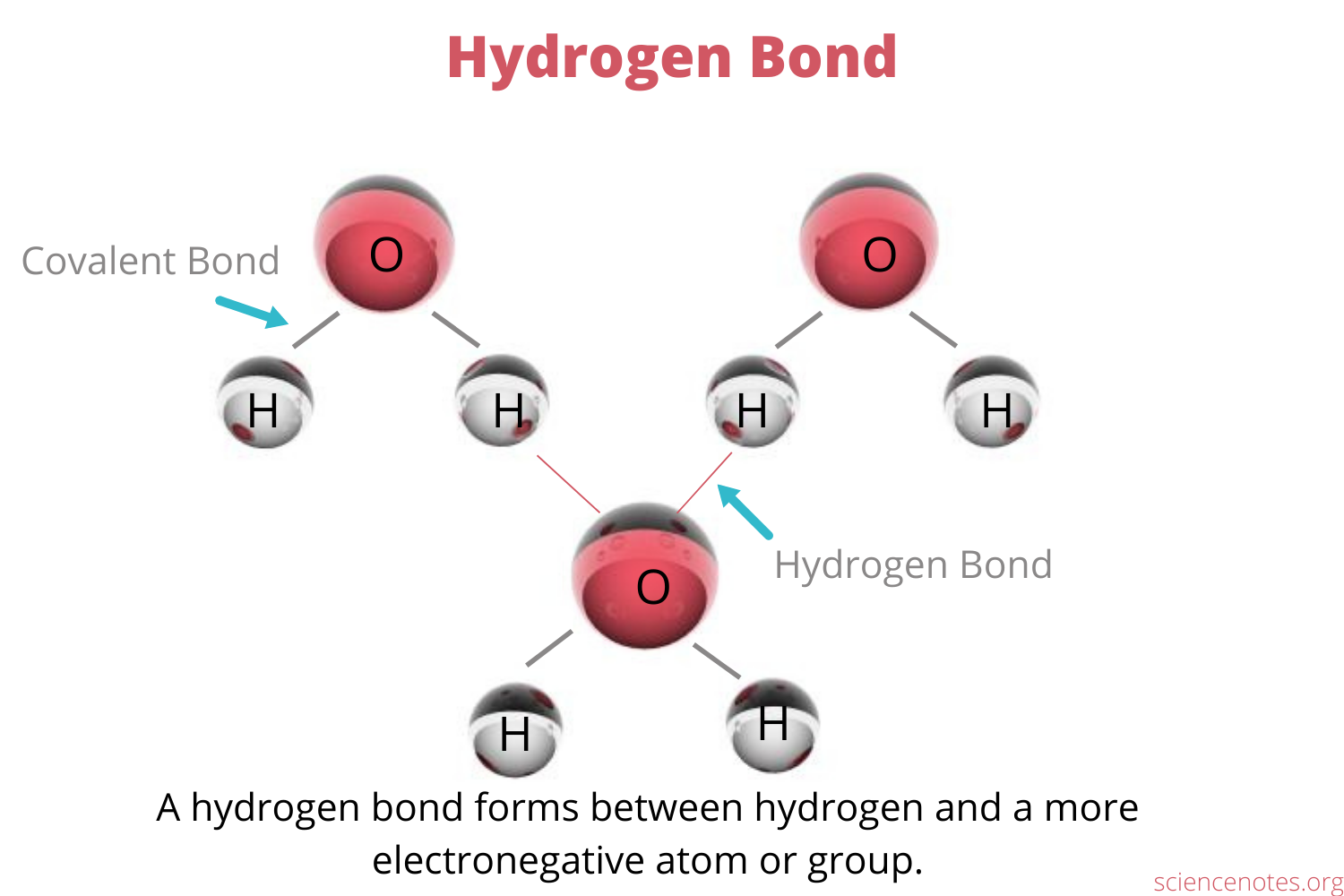
Van der Waals interactions
Chemical reactions, which are the making/breaking of the composition of matter(ex, reaction of hydrogen and oxygen to make water)

When writing a chemical reaction, we use arrows to tell the conversion of the starting material(hydrogen and oxygen) called reactants to the products(the water molecules)( the coefficients indicate the # of molecules involved )
Matter in a reaction cannot be made or destroyed only rearranged
Some chemical reactions can reverse the reaction( hydrogen and nitrogen can combine to make ammonia, and ammonia can decompose into nitrogen and hydrogen)
Factors that affect the rate of reaction is the concentration of reactants, the higher the concentration of reactant molecules, the more often they collide with each other. This also holds true for the products
The more collisions that a product gets the reverse reaction
chemical equilibrium is the state in which the forward reaction equals the rate of the backward reaction
Water to life
Molecules: Structure and function
A lot of the chemicals that make living stuff are based on carbon
All of the molecules such as proteins and DNA are carbon-based
Common stuff that makes life is hydrogen, oxygen, nitrogen, sulfur, and phosphorus
Organic chemical is the study of carbon compounds
Organic pounds could be simple, like methane or carbon dioxide, to proteins, which are made up of 100,000 atoms
carbon doesn’t really like to lose or gain electrons to make ionic bonds, it would rather do this by giving or getting 4 electrons to do so.
But carbon would much rather complete its shell by sharing with another atom and have 4 covalent bonds
Carbon chains make skeletons of most organic molecules.
-these “carbon skeletons” can vary in length, branching, bonds(where a double bond is put), and rings(which is another way to show the structure of carbon skeletons)
Hydrocarbons are organic molecules which are only made up of carbon and hydrogen
Hydrogen is attached to carbon skeletons when there is room for covalent bonding(it fills in any other valence left on the carbon)
Fats are mostly hydrocarbon with an attached part which is nonhydrocarbon
fats are hydrophobic because of the bonds between carbon and hydrogen, which are nonpolar covalent bonds( They share electrons equally)
Hydrocarbon stores large amounts of energy
Isomers are compounds that have the same molecular formula(same parts) but have different structures which leads to them having different properties
-There are 3 types of isomers: Structural, Geomtric, and Enantiomers.
Structural isomers have their atoms rearranged
with a higher carbon skeleton the higher amounts of variants there will be
Geometric isomers have the same covalent partnership but have different spatial placements
Enantiomers are molecules that are mirror images of each other
A middle carbon is called “asymmetric carbon” because it is attached to 4 different atoms/groups of atoms
Functional groups are groups which are in a molecule and have their proprties even without other atoms being in there
they replace 1 or more hydrogens which are bonded to the carbon skeleton of a hydrocarbon
There are 6 functional groups: Hydroxyl, Carbonyl, Carboxyl, Animo, Sulfhydryl, Phosphate
The Hydroxyl group is a hydrogen bonded to oxygen which is also bonded to the carbon skeleton of a molecule
Compounds with a hydroxyl are called Alcohols (their names usually end in -ol)
The structural formula is (-OH or -HO)
The hydroxyl group is polar, because of this, the oxygen atom brings electrons close to itself and water molecules are attached to the hydroxyl group
And this helps with dissolving
The Carbonyl group is a carbon atom which is bonded to oxygen with a double bond
if it is at the end of a skeleton, the resulting organic compound will be called “aldehyde”
it not it will called “ketone”
The Carboxyl group is when an oxygen atom is double-boned with carbon, which is also bonded with a hydroxyl group( the structure is “-cooh”
Carboxylic acids are compounds that have a carboxyl group
the reason why carboxyl groups have acidic properties is that they can be a source of hydrogen ions, since the bond between oxygen and hydrogen is very polar, the hydrogen tends to just dissociate reversibly
Amino groups are made up of a nitrogen atom which is bonded with two hydrogen atoms to the carbon skeleton
compounds with amino groups are called “Amines”
sulfhydryl groups are made up of an atom of sulfur bonded to an atom of hydrogen
compounds with sulfhydryl groups are called “thiols”
Phosphate groups are made up of 2 anion oxygens, double-bonded oxygen, and a final oxygen
There are four main types of biological molecules: lipids, proteins, carbohydrates, and nucleic acids
Carbohydrates, lipids, and proteins are chainlike molecules; they are called polymers
polymers are big/long chians ade up of the same parts which are also linked to eachother by covalent bonds
The molecules which make up the polymers are called “monomers”-they are just
it goes monomer, polymer, Marcomolecule
Monomers are actechied together by a reaction which covanetly bonds them because of the loss of water
this reaction is name the condensation reaction or for the more info name “dehydration reaction"
within this bond both of molecules give apart of water to from a full water molecule( one gives a hydroxyl group and a hydreogen
this process is only done with the help of Enzymes(speical proteins which help speed up chemcial reactions in cells)
Hydrolysis is the revsres reaction of the hydration reations
The bonds between are broken down when water is added
A hydroxyl group go one monomer and a hydrogen goes to the other monomer
An example of this reaction happening is in our bodies. When we eat food the polymers of the food are way to big for us bodies to break down, so with enzymes our bodies break down and distrbute the now broken polymers.
The main thing that makes building nearly an endless list of polymers is the arrangment of the polymers
Carbohydrates are sugar molecules(aka they are made up of sugars)
the simpest type of carbphydrate are own as “monosacchardies"- 1 sugar
“Disaccharides” are dobule sugars- they are made up of 2 monosaccharides
they are joined by water
Any type of marcomolecule which is a carbohydrate is called “polysaccharide"- many sugars
For the most part monoscaacharies have the molecular formula of “CH2O"
C6H12O6 has big importnace in life
This monosacchirde has a carbonyl group and alot of hydroxyl groups, and depending on where the carbonyl is it can either be an “aldehyde" or an “Ketone” sugar
Aldehde is a carboyl with a hydrogen attched to it
Kentone is a carboyl without a hydrogen but has other groups or molecules attched to it
Most sugars end with -"ose"
An exmaple of boh Aldehyde and Kentose are Glucose(aldoses) and Fructose(Ketoses)-Fructose is also a structural isomer of glucose
Another way of classifing sugars is by looking at the carbon skeleton, it usally ranges from 3 to 7 cabons long
Exmaple is Glucose which has a carbon skeleton of 6( this is called Hexoses)
for most solutions which have water, molecules tend to form rings
Celluar respitation cells get enegry which is stored in glucose molecules
As well as being the great soucre of enegry for celluar work, their carbon skeleton can be used for other types of small molecules such as animo acids and fatty acids( before this they are turn into poly or monosacchardies)
Disaccharides which are made up of 2 monosacchardies, are joined together by something called “glycosidic linkage"- A covalent bond whch forms between the monosacchardies through the dehydration reacation
Maltose is a Disaccharide which formed by linking the 2 molecules of glucose
Polysacchires are marcomoles which have 100s to 1000’s of monosacchardies joined together(through of course glycosidc linkage)
there function is detemined by type of monosacchardies they hve and the postion of the glycosdid linkages
Polysaccharides can function as buildig material or stroage material
Starch is a stroage Polysaccharide and its monomers are only made up of glucose
most of the monomers within the starch molecule are bonded together by 1-4 linkages(the 1st carbon of amonomer and the 4th carbon of the other monomer
the simpest form of starch is called “amylose” it is an unbranched from of Amylopection
Amylopection is more complex, it had branches and is link with 1-6 linkages at the branch points
Plants like to strach as granules in cell structures called “plastids" and chlotoplasts
Animals store Polysaccharides in a form similar to amylopection but it is more branched, it is called “Glycogen”
in animals we store it in out livers and muslces, but we go throuogh it very quickly meaning we need to eat food to keep them up
Cellouse is a structural polysaccharide which is only in plants, they build what is known as the cells walls of the plant
cellouse is a polymer of glucose, the main difference is the glylcosidic linkages.
in gluocse there is 2 differnet ring strctures for glucose
when forming the ring there is two differnet postions for the hydroxyl group attched to the # 1 carbon to be in
The postion of the hydroxyl could be below or above the plane of the ring
when is it below it is known as “Alpha” and when its below its known as “beta” gluocse
For exmaple in strach all the glucose molecules are in the alpha postion
while the cellose molecule is in the beta postion( this would make every other monomer upside down)
The cellouse molecule is hecial(meaning in the shape of a helix-like DNA), its hydroxyl groups can hydrogen bond with other hydroxyls of another cellouse lying paraellel to it, when they attched this way and grouped, these groups are called mircofibrils”
these are strong cable like structures
Emzyes which can break down strach by hydrolyzin the alpha linkages cannot break down the beta linkages of something like cellouse
Chitin is another important structural polysaccharide, this is used for build the exoskeleton of most insects and other related animals
its quite similar to cellouse but it a nitrogen group instead of a hydrocarbon
lipids, the only class of marcomolecules which do not have a polymer
the most important trait of the lipid is that they are hydrophobic/ have little affinity for water
they may be a little polar, but they almost constiti of hydrocabons(To explain quicky, since carbon and hydrogen are very simialr in electronegativtity, they same electrons evenly(nonpolar bonds) and they have no space for water)
types of lipids include: waxes, (some)pigments, fats, and steroids.
fats are made up of two things: glycerol and fatty acids
glycerol is an alcohol which is made of 3 carbons which has hydroxyl groups
Fatty acid has a long 16-18 carbon skeleton, at the end of it has carboxyl group(which a functional group which is where the name comes from)
attched to the carboxyl is a long hydrocarbon chain( the bonds with carbon and hydrogen are the reason they are hydrophobic
when it froms, 3 fatty acids meet a glycerol and bond by something called “ester linkages”(formed bonds between a carboxyl and hydroxyl"), the resulting fat is called “triacylglycerol”
When it is done forming the result leads to 1 head(glycerol) and 3 tails(fatty acids)
There are 2 types of triglycerides: Saturated(without double bonds in the fatty acids allowing a lot of extra hydrocarbons to addon), unsaturated(with double bonds limting the amount of hydrocarbonds)
if the tail have a kink that is where a double bond is
the big functions of fats are enegry stroage( such as gas, hydrocarbon chains are rich in enegry
Fats stroage 2x as much as a Polysaccharide like strach
(the main reason is because they dont move and dont need such a big stroage of fat
they stroage this extra engery in adiposr cells, they can swell ot shirnk depending on if fat is being deposited or withdrawn
Phospholipids simailr to triglycerides with the main difference being they have only 2 tails(fatty acids) and the missing tail is filled in by Phosphate group.
the tails are hydrophobic and the head is hydrophilic, when put into wwater they form a type of cicrcle shape named a “Micelle”
when this happens is forms a type of “bilayer"(double layer)
The head of the phospholipid is out facing the water while the tail is facing the inside of the micelle
An example of this function is in cells, where the cell membrane in made of phospholipds to keep water outside the memebrane
Steroids are lipids which there carbon skeleton is 4 rings of carbon
Steroids differ mainly because of there functional group
Cholesterol is a steroid, its a common part in animals cells but is also a percuror to where alot of the steroids are made/synthesized
A lot of sex horomes are made from steroids( another name for them are sex steroids
Proteins are used for a lot of things such as: Structural support, stroage, transport of other substances, signaling, defence, movement, and enzymes
Each kind of protein has there own unqine 3d shape
Proteins polymers are called “polypeptides”, these polypeptides are made up of things called “ amino acids” (Amino acids[AA], polypeptides, Proteins)
There are only 20 sets of amino acids
Proteins are made up of 1 or more polypeptides which are folded in specific manger
Amino acids are made up of a carboxyl group, amino group, a hydrogen atom, and a variable group also called an “R group”(because it is symbol is an R)(another name for them is called “side chain”)
The side chain determines the physical and chemical properties of the amino acid
Amino acids are grouped together by proptries of the side chains
there are 3 kinds of groups: Polar, Non polar, Electrically charged
non polar are hydrophobic
Polar are hydrophilic
electrically charged are split into to kinds: Acidic and basic
Acidic side chains have this proptrie due to there being a carboxyl group(which is usually ionized or dissociated at a cellular ph)
Basic amino have amino groups in there side chains which are usually positive charged
both basic and acidic are hydrophilic
Peptide bonds are formed when the carboxyl group is adjacent to the amino group of amino acids (they can be helped by enzymes, its also a type of covalent bond), repeated over and over it makes polypeptides
Statistics (mini unit)
Statistics: The study of collecting and analyzing data
Scientists usually get data on samples of a population
- this is meant to infer things about the population instead of looking at the whole population
The first step in analysis is to graph the data and examine the distribution
-usually, the data will show a “normal distribution” of the data

Measures of Central Tendencies:
-quick note when working with data sets make sure that you are ordering them from smallest to lowest to make it easy
3 M’s of data (mean, median, mode,) | Formula | Example |
Mean: The average of a data set | total # / # of samples | 4+4+2+10+9=29/5=5.8 |
Median: The middle of a data set | If odd, then the middle is the median If even, then it’s both middle # divided by 2 | (1,2,4,6,8): 4 is the median (1,2,6,7,3,4): 6+7/2 = 6.5 is the median |
Mode: the # or thing that happens most often | None | (1,2,3,4,4,4,5,6) 4 happens the most amount of times |
Range: the difference between the highest and lowest # | Highest # - lowest # | (1,2,9,8,7) 9(highest) - 1(lowest) =8, there is a difference of 8 between 1 and 9 |
Things about the Range
The bigger/larger the range the greater the spread of data
the smaller the range the less the data will spread
with is often used with standard deviation
Measure of Variability:
Variability: How far the data goes away from the central tendency
Standard Deviation: How spread out the data is from the mean
Low Standard deviation( The good): The data is closer to the mean
The MV or independent variable is likely to affect it
High Standard deviation( The bad): The data is more spread out from the mean
Factors other than an MV are likely to affect it
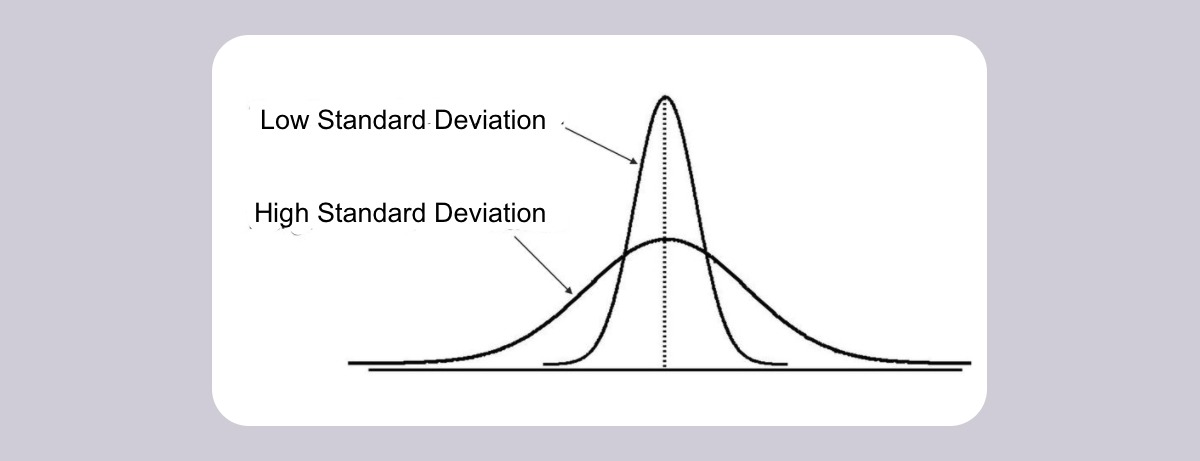
Deviations from the mean
1 deviation from the mean on the x-axis, either way, shows 68% of the data
2 deviations from the mean on the x-axis either way shows 95% of the data
3 deviations from the mean on the x-axis either way shows 99%of the data
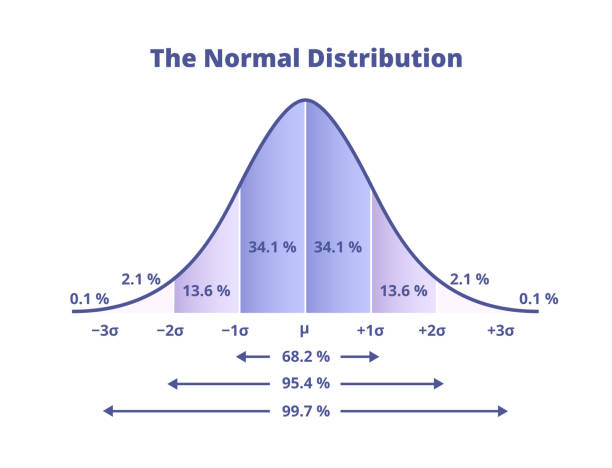
Must know | formula | example |
±, this meant to show deviation in either direction | ± σ (every time you deviate one more it’s x(σ) where x is the amount you want to deviate | ±7.45 |
Standard Deviation | σ=√[(∑ x - μ)² /n-1] | (65,52,71,56,61)= 305/5=61, 65-61=(4)²=16 52-61=(-9)²=81 71-61=(10)²=100 56-61=(-5)²=25 61-61=(0)²=0 16+81+100+25+0=222 5-1= 4(units of freedom) √[(222)/4]= 7.45 |
Units of freedom: this is the divisor for standard deviation | n(total # of samples) - 1 | 5-1= 4 |
Standard error of the mean: how far the sample mean of the data is likely to be from the true population
it is based on the standard deviation number
With a high standard error, you get more confidence in the data
with a lower standard error, you get less confidence in the data
The most common one to see is 95% as ±2 SE
The formula is SE= S/√n (where S is the standard deviation and n is the sample size)
Error bars: a graphical representation of uncertainty or variability of data in a graph( this is based on Standard deviation, Standard error of the mean)

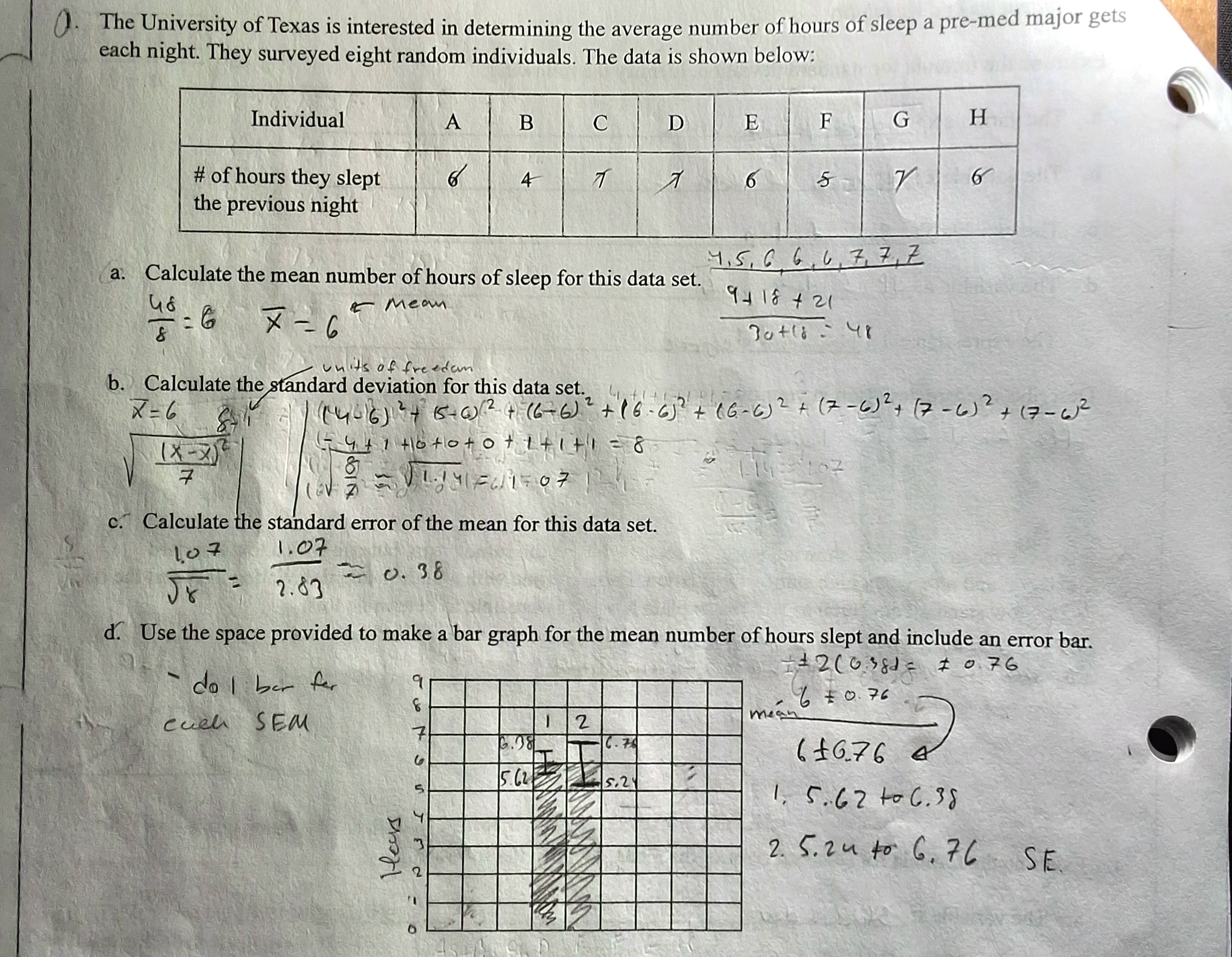
Example problem with everything
Unit 2: Cell structure and their functions
The cell is the smallest unit of living organism/ unit of life
Cell theory(older) | Cell theory (Modern | |
|
| |
The main thing that determines the function of a cell is its shape ( there is a structure-function relationship)
The main reason why cells have a certain limit size is b/c the cell needs to be able to pass food, gases, and other things(When the cell is bigger it can’t do so)
The cell ratio is: “volume: surface area” If a cell were to grow quickly the volume would grow quicker than the surface area and it wouldn’t allow things to move through the cell fast enough for the cell’s needs\
But in some cases, big cells have ways of dealing with the size
white blood cells grow more nuclei to get enough protein and RNA
Active cells have a lot of folds on them to increase the surface area ( on their membrane)\
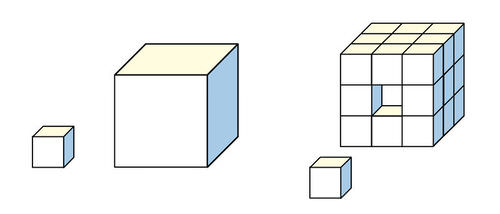
Think about cells like these cubes, the one to the right has less surface area than the one to the left
more surface area means exposure to an environment
the main reason why cells can’t grow so big is because of a law called 'the “cubic square law”, this law states that as a shape grows in size, its volume increases faster than its surface area. This leads to a situation where the surface area is insufficient to support the metabolic needs of the cell, limiting its size and overall efficiency.