
Chapter 10 Study Guide
10.1 LOs
State the main goal of antimicrobial treatment.
destroy the infective agent without harming the host’s cells
Identify the sources for the most commonly used antimicrobials.
from bacteria: Streptomyces and Bacillus
from molds: Penicillium and Cephalosporium
Describe two methods for testing antimicrobial susceptibility.
Kirby-Bauer technique: agar diffusion test that provides useful data on antimicrobial susceptibility
the surface of a plate of special medium is spread with test bacterium and small discs with premeasured amount of antimicrobial are dispensed onto the bacterial lawn
after incubation, the zone of inhibition is measured and compared with a standard for each drugs
E-test: uses a strip to produce the zone of inhibition
the strip contains a gradient of drug-calibrated in micrograms so that the MIC can be measured by observing the mark on the strip that corresponds to the edge of the zone of inhibition
tube dilution tests: antimicrobial is diluted serially in tubes of broth and then inoculated with a small sample of pure culture, incubated, and examined for growth (turbidity)
minimum inhibitory concentration (MIC): the smallest concentration of drug needed to visibly control microbial growth
Define therapeutic index, and identify whether a high or low index is preferable.
therapeutic index: the ratio of toxic dose to effective therapeutic dose, used to assess the safety and reliability of the drug
low index = greater potential for toxic reactions
high index = wider margin of safety
10.2 LOs
Explain the concept of selective toxicity.
selective toxicity: property of drugs meaning that they should kill or inhibit microbial cells without simultaneously damaging host tissues
List the five major targets of antimicrobial agents.
inhibition of cell wall synthesis
inhibition of DNA/RNA structure & function
inhibition of ribosome in protein synthesis
interference with cytoplasmic membrane structure & function
inhibition of folic acid synthesis
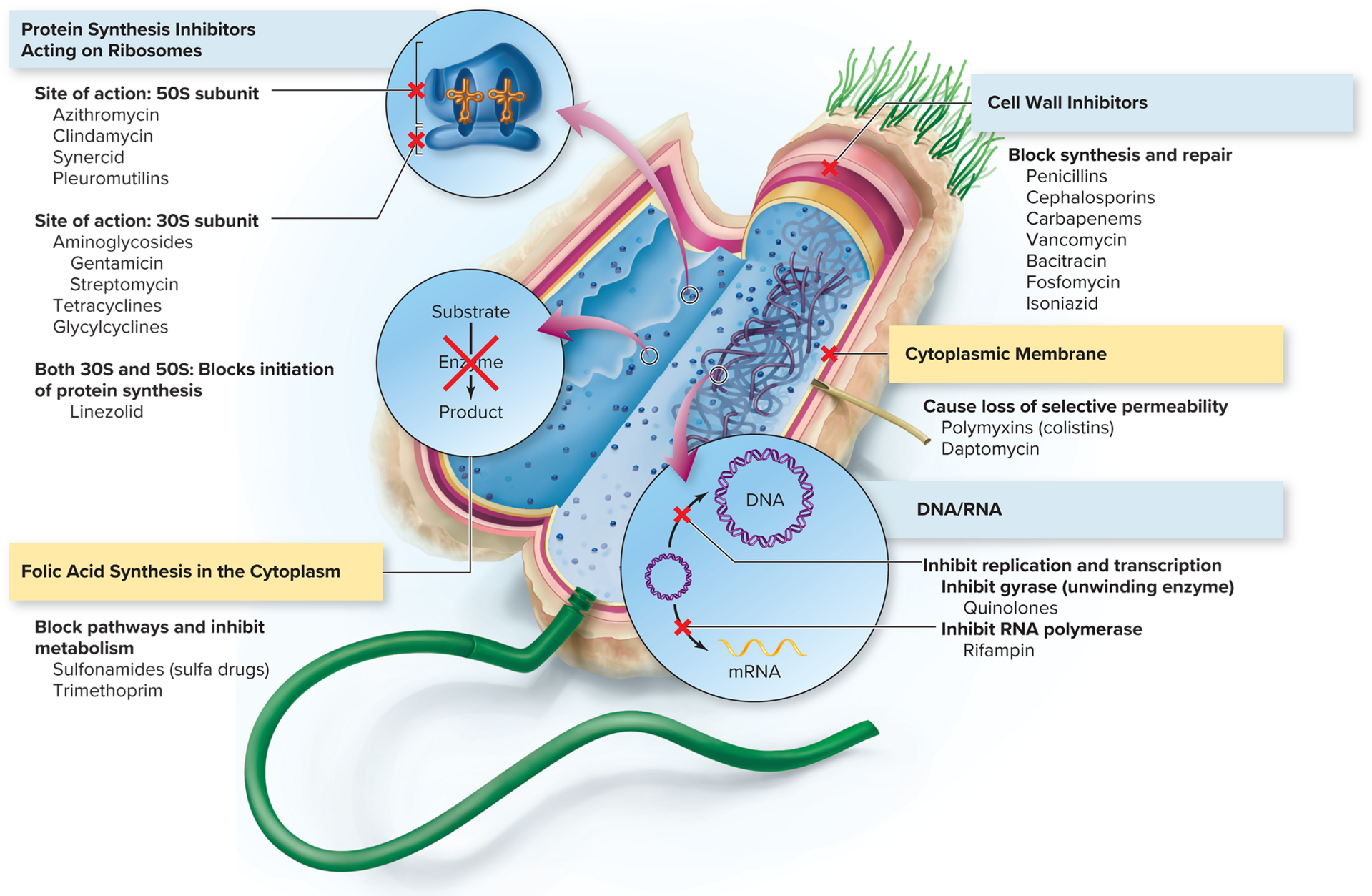
Identify which categories of drugs are most selectively toxic and why.
those that target structures common to the infective agent and host cell (ex: the cytoplasmic membrane)
Distinguish between broad-spectrum and narrow-spectrum antimicrobials, and explain the significance of the distinction.
broad-spectrum: effective against more than one group of bacteria
narrow-spectrum: target a specific group of bacteria
Identify the microbes against which the various penicilins are effective.
penicillins G & V: gram + cocci, some gram - (meningococci, syphilis, spirochetes)
ampicillin, carbenicillin, amoxicillin: gram - enteric rods
nafcillin, cloxacillin: penicillinase-producing bacteria
clavulanic acid: inhibits beta-lactamase enzymes, added to penicillin to increase effectiveness against penicillinase-producing bacteria
Explain the mode of action of penicillinases and their role in treatment decisions.
penicillin G: narrow spectrum, best used when bacteria are sensitive, low cost & low toxicity
penicillin V: narrow spectrum, good intestine absorption
methicillin, nafcillin: narrow spectrum, not typically susceptible to penicillinase
ampicillin: broad spectrum, works on gram - bacilli
amoxicillin: broad spectrum, works on gram - infections, good absorption
azlocillin, mezlocillin, ticarcillin: very broad spectrum, effective against Pseudomonas, lower toxicity than aminoglycosides
Identify antimicrobials that act by inhibiting protein synthesis.
aminoglycosides: broad spectrum; treat gram - rods, some gram + bacteria, bubonic plague, tularemia, tuberculosis
tetracyclines: treats gram +/- rods & cocci, aerobic/anaerobic bacteria, mycoplasmas, rickettsias, spirochetes
glycylcyclines: effective against tetracycline-resistant bacteria
macrolides: broad spectrum; treat ear/respiratory/skin infections, Mycobacterium infections, AIDS patients
Explain how drugs targeting folic acid synthesis work.
they block enzymes required for tetrahydrofolate (needed by cells for folic acid synthesis and DNA/RNA/amino acid production) synthesis
Identify one example of a fluoroquinolone.
ciprofloxacin, ofloxacin, levofloxacin
Describe the mode of action of drugs that target the cytoplasmic or cell membrane.
interact with membrane phospholipids, distort the cell surface & cause leakage of protein and nitrogen bases (in gram - bacteria)
Discuss how treatments of biofilm and nonbiofilm infections differ.
treatments of biofilm infections involve impregnating biomaterials with antibiotics prior to insertion, adding DNase or daptomycin to antibiotics
Name the four main categories of antifungal agents.
macrolide polyenes
azoles
echinocandins
allylamines
Explain why antiprotozoal and antihelminthic drugs are likely to be more toxic than antibacterial drugs.
they have many similarities to human physiology
List the three major targets of action of antiviral drugs.
barring penetration of virus into host cell
blocking transcription/translation
preventing maturation of viral particles
10.3 LOs
Discuss two main ways that microbes acquire antimicrobial resistance.
spontaneous mutations in critical chromosomal genes
acquisition of entire new genes or sets of genes via horizontal transfer from another species.
List five cellular or structural mechanisms that microbes use to resist antimicrobials.
new enzymes are synthesized, inactivating the drug once new genes are acquired
permeability/uptake of drug into bacterium is decreased (via mutation)
drug is immediately eliminated through the acquisition of new genes
binding sites for drugs are decreased in number or affinity (via mutation or new gene acquisition)
affected metabolic pathway shuts down or alternative pathway is used (via mutation of original enzymes)
Discuss at least two novel antimicrobial strategies that are under investigation.
RNA interference: attempting to use small pieces of RNA to shut down the metabolism of pathogenic microbes
defense peptides: peptides are used to target membranes and other cell structures to kill drug-resistant bacteria
CRISPR: can cut genes that are resistant to antibiotics
drugs from noncultivable bacteria: are unable or slow at developing drug resistance
bacteriophages: an advantage is their extreme specificity
10.4 LOs
Distinguish between drug toxicity and allergic reactions to drugs.
allergic reactions: occurs because the drug acts as an antigen and stimulates an allergic response
drug toxicity: foreign chemicals or toxins harm the host’s tissues or cells
Explain what a superinfection is and how it occurs.
superinfection: occurs due to an overgrowth of drug-resistnat microorganisms when antimicrobial therapy destroys beneficial resident species of the normal biota
10.1 LOs
State the main goal of antimicrobial treatment.
destroy the infective agent without harming the host’s cells
Identify the sources for the most commonly used antimicrobials.
from bacteria: Streptomyces and Bacillus
from molds: Penicillium and Cephalosporium
Describe two methods for testing antimicrobial susceptibility.
Kirby-Bauer technique: agar diffusion test that provides useful data on antimicrobial susceptibility
the surface of a plate of special medium is spread with test bacterium and small discs with premeasured amount of antimicrobial are dispensed onto the bacterial lawn
after incubation, the zone of inhibition is measured and compared with a standard for each drugs
E-test: uses a strip to produce the zone of inhibition
the strip contains a gradient of drug-calibrated in micrograms so that the MIC can be measured by observing the mark on the strip that corresponds to the edge of the zone of inhibition
tube dilution tests: antimicrobial is diluted serially in tubes of broth and then inoculated with a small sample of pure culture, incubated, and examined for growth (turbidity)
minimum inhibitory concentration (MIC): the smallest concentration of drug needed to visibly control microbial growth
Define therapeutic index, and identify whether a high or low index is preferable.
therapeutic index: the ratio of toxic dose to effective therapeutic dose, used to assess the safety and reliability of the drug
low index = greater potential for toxic reactions
high index = wider margin of safety
10.2 LOs
Explain the concept of selective toxicity.
selective toxicity: property of drugs meaning that they should kill or inhibit microbial cells without simultaneously damaging host tissues
List the five major targets of antimicrobial agents.
inhibition of cell wall synthesis
inhibition of DNA/RNA structure & function
inhibition of ribosome in protein synthesis
interference with cytoplasmic membrane structure & function
inhibition of folic acid synthesis

Identify which categories of drugs are most selectively toxic and why.
those that target structures common to the infective agent and host cell (ex: the cytoplasmic membrane)
Distinguish between broad-spectrum and narrow-spectrum antimicrobials, and explain the significance of the distinction.
broad-spectrum: effective against more than one group of bacteria
narrow-spectrum: target a specific group of bacteria
Identify the microbes against which the various penicilins are effective.
penicillins G & V: gram + cocci, some gram - (meningococci, syphilis, spirochetes)
ampicillin, carbenicillin, amoxicillin: gram - enteric rods
nafcillin, cloxacillin: penicillinase-producing bacteria
clavulanic acid: inhibits beta-lactamase enzymes, added to penicillin to increase effectiveness against penicillinase-producing bacteria
Explain the mode of action of penicillinases and their role in treatment decisions.
penicillin G: narrow spectrum, best used when bacteria are sensitive, low cost & low toxicity
penicillin V: narrow spectrum, good intestine absorption
methicillin, nafcillin: narrow spectrum, not typically susceptible to penicillinase
ampicillin: broad spectrum, works on gram - bacilli
amoxicillin: broad spectrum, works on gram - infections, good absorption
azlocillin, mezlocillin, ticarcillin: very broad spectrum, effective against Pseudomonas, lower toxicity than aminoglycosides
Identify antimicrobials that act by inhibiting protein synthesis.
aminoglycosides: broad spectrum; treat gram - rods, some gram + bacteria, bubonic plague, tularemia, tuberculosis
tetracyclines: treats gram +/- rods & cocci, aerobic/anaerobic bacteria, mycoplasmas, rickettsias, spirochetes
glycylcyclines: effective against tetracycline-resistant bacteria
macrolides: broad spectrum; treat ear/respiratory/skin infections, Mycobacterium infections, AIDS patients
Explain how drugs targeting folic acid synthesis work.
they block enzymes required for tetrahydrofolate (needed by cells for folic acid synthesis and DNA/RNA/amino acid production) synthesis
Identify one example of a fluoroquinolone.
ciprofloxacin, ofloxacin, levofloxacin
Describe the mode of action of drugs that target the cytoplasmic or cell membrane.
interact with membrane phospholipids, distort the cell surface & cause leakage of protein and nitrogen bases (in gram - bacteria)
Discuss how treatments of biofilm and nonbiofilm infections differ.
treatments of biofilm infections involve impregnating biomaterials with antibiotics prior to insertion, adding DNase or daptomycin to antibiotics
Name the four main categories of antifungal agents.
macrolide polyenes
azoles
echinocandins
allylamines
Explain why antiprotozoal and antihelminthic drugs are likely to be more toxic than antibacterial drugs.
they have many similarities to human physiology
List the three major targets of action of antiviral drugs.
barring penetration of virus into host cell
blocking transcription/translation
preventing maturation of viral particles
10.3 LOs
Discuss two main ways that microbes acquire antimicrobial resistance.
spontaneous mutations in critical chromosomal genes
acquisition of entire new genes or sets of genes via horizontal transfer from another species.
List five cellular or structural mechanisms that microbes use to resist antimicrobials.
new enzymes are synthesized, inactivating the drug once new genes are acquired
permeability/uptake of drug into bacterium is decreased (via mutation)
drug is immediately eliminated through the acquisition of new genes
binding sites for drugs are decreased in number or affinity (via mutation or new gene acquisition)
affected metabolic pathway shuts down or alternative pathway is used (via mutation of original enzymes)
Discuss at least two novel antimicrobial strategies that are under investigation.
RNA interference: attempting to use small pieces of RNA to shut down the metabolism of pathogenic microbes
defense peptides: peptides are used to target membranes and other cell structures to kill drug-resistant bacteria
CRISPR: can cut genes that are resistant to antibiotics
drugs from noncultivable bacteria: are unable or slow at developing drug resistance
bacteriophages: an advantage is their extreme specificity
10.4 LOs
Distinguish between drug toxicity and allergic reactions to drugs.
allergic reactions: occurs because the drug acts as an antigen and stimulates an allergic response
drug toxicity: foreign chemicals or toxins harm the host’s tissues or cells
Explain what a superinfection is and how it occurs.
superinfection: occurs due to an overgrowth of drug-resistnat microorganisms when antimicrobial therapy destroys beneficial resident species of the normal biota