2.1-2.3 Mixtures Solutions and Solvent (Experimental Techniques)
A mixture contains more than one substances. The substances are just mixed together not chemically combined.
- air is a mixture of oxygen, nitrogen and small amounts of other gases
- shampoo is a mixture of several chemicals and water.
Solutions: When you mix sugar and water, the sugar seems to disappear. That us because its particles spread all through the water particles like this:
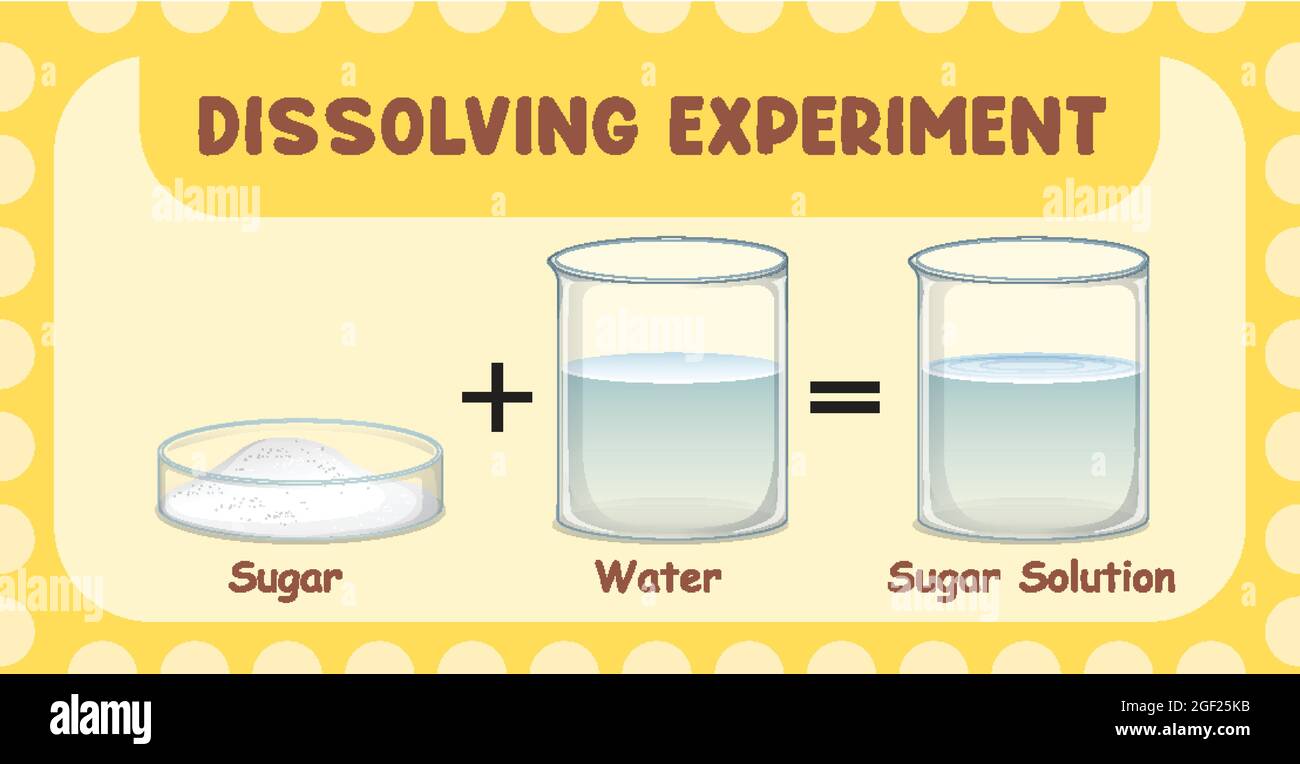
The sugar has Dissolved in the water, giving a mixture called a solution. Sugar is the solute, water is the solvent.
- A soluble solid usually gets more soluble as the temperature rises. A solution is called saturated when it can dissolve no more solute at its temperature. Sugar is more soluble in hot water than in cold water.
- A volatile liquid is one that evaporated easily this is a sign that the forces between its particles are weak so volatile liquids have low boiling points too.
Pure substances and Impurities
- %%A pure substances has no other substances mixed in with it.%%
- Often it doesn’t matter is a substance is not pure. We wash in tap water without thinking much. But sometimes purity is very important if you are making a new medical drug or a flavoring for for you must make sure it contains nothing that could harm people.
- %%An unwanted substance mixed with the desirable substance is called an impurity%%
How can you tell something is pure?
Chemists sometimes use complex method to check for purity. But there is one simple method you can use in the lab: You can check melting and boiling points
A pure substance has a definite, sharp, melting point and boiling point. These are difference for each substance
When a substance contains impurity the melting point falls and boiling point rises
it melts and boils over a range of temperatures, not sharply.
The bigger the impurity there is:
the bigger the change in melting and boiling points
the wider the temperature range over which melting and boiling occur.
Separation Method 1
@@Filtering@@
^^used to separate an insoluble or undissolved substance in water so it is easy to separate in the filter paper.^^ example chalk and water, the chalk is trapped in the filter paper, while the water passes through the trapped solid is called residue. the water is the filtrate.
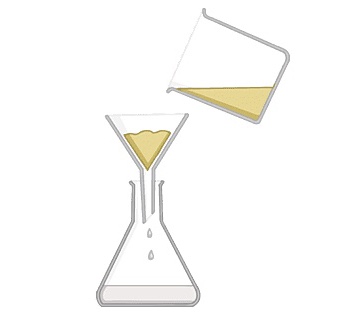
Filter paper is placed in a filter funnel above another beaker
The mixture of insoluble solid and liquid is poured into the filter funnel
Filter paper will only allow small liquid particles to pass through in the filtrate
Solid particles are too large to pass through the filter paper so will stay behind as a residue
==Crystallization==
You can obtain many solids from their solutions by letting crystals form. The process is called Crystallization. It works because the soluble solids tent fu be less sikh em af lower temperatures.
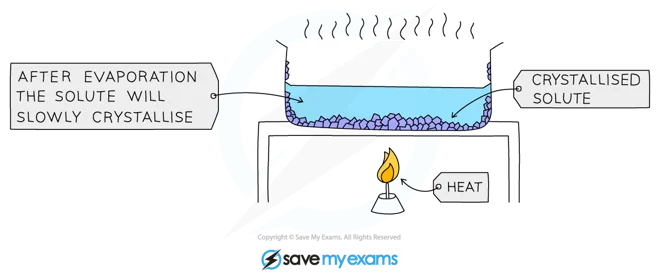
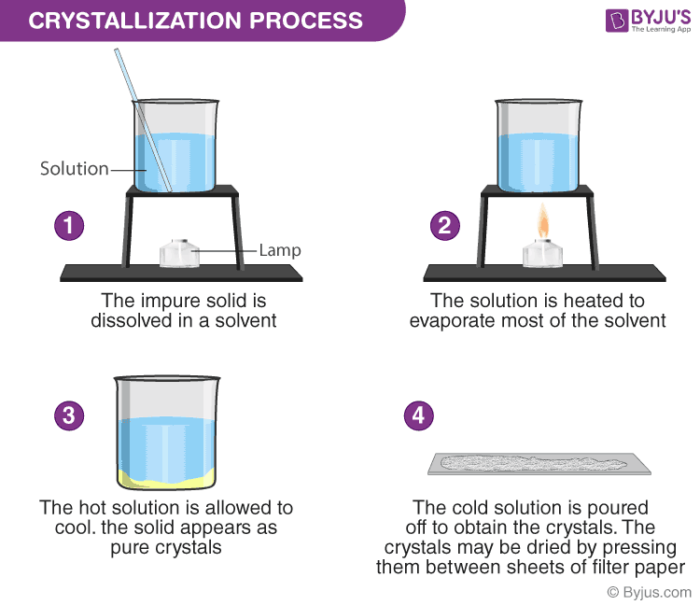
^^Example and procedure^^ : Used to separate a dissolved solid from a solution, when the solid is more soluble in hot solvent than in cold (e.g. copper sulphate from a solution of copper (II) sulphate in water)
The solution is heated, allowing the solvent to evaporate and leaving a saturated solution behind
You can test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
- If the solution is saturated, crystals will form on the glass rod when it is removed and allowed to cool
The saturated solution is allowed to cool slowly and solids will come out of the solution as the solubility decreases, and crystals will grow
Crystals are collected by filtering the solution
They are then washed with distilled water to remove any impurities and then allowed to dry.
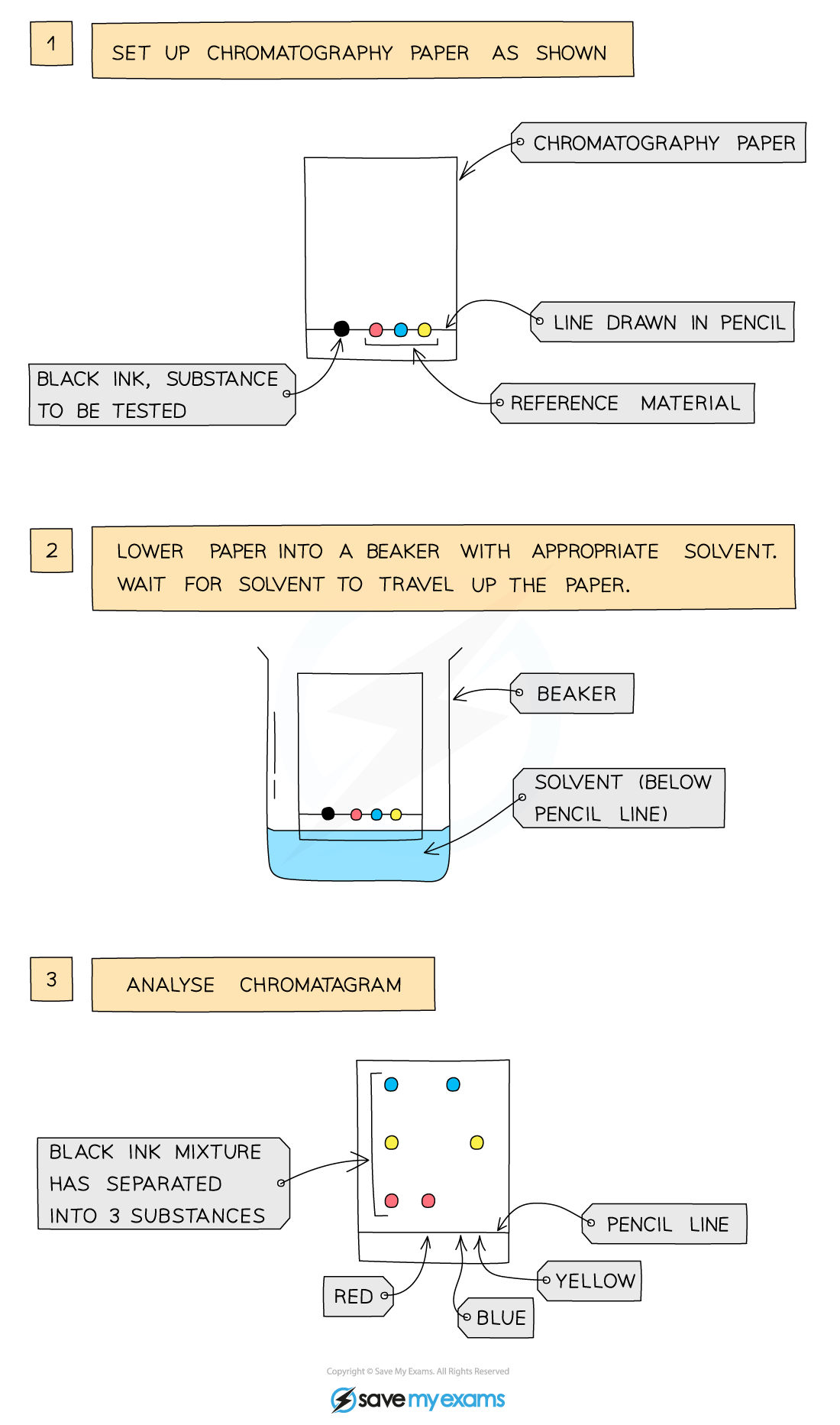
Paper Chromatography
This technique is used to separate substances that have different solubilities in a given solvent (e.g. different coloured inks that have been mixed to make black ink)
it is also used to separate a mixture of substances
A pencil line is drawn on chromatography paper and spots of the sample are placed on it, why? =
Pencil is used for this as ink would run into the chromatogram along with the samples
The paper is then lowered into the solvent container, making sure that the pencil line sits above the level of the solvent so the samples don´t wash into the solvent container
The solvent travels up the paper by capillary action, taking some of the coloured substances with it
Different substances have different solubilities so will travel at different rates, causing the substances to spread apart. Those substances with higher solubility will travel further than the others
This will show the different components of the ink / dye
Simpler way to explain paper chromatography is:
1- place a drop of black ink in the center of some filter paper. let it dry. then add 3-4 more drops on the same spot in the same way
2- Now drop water onto the ink spot, one drop at a time. the u m slowly spreads out and separates into rings of different colors.
3- suppose there are three rings yellow, red
and blue thus shows that ink contained three dyes coloured yellow red and blue.
Separation Method 2
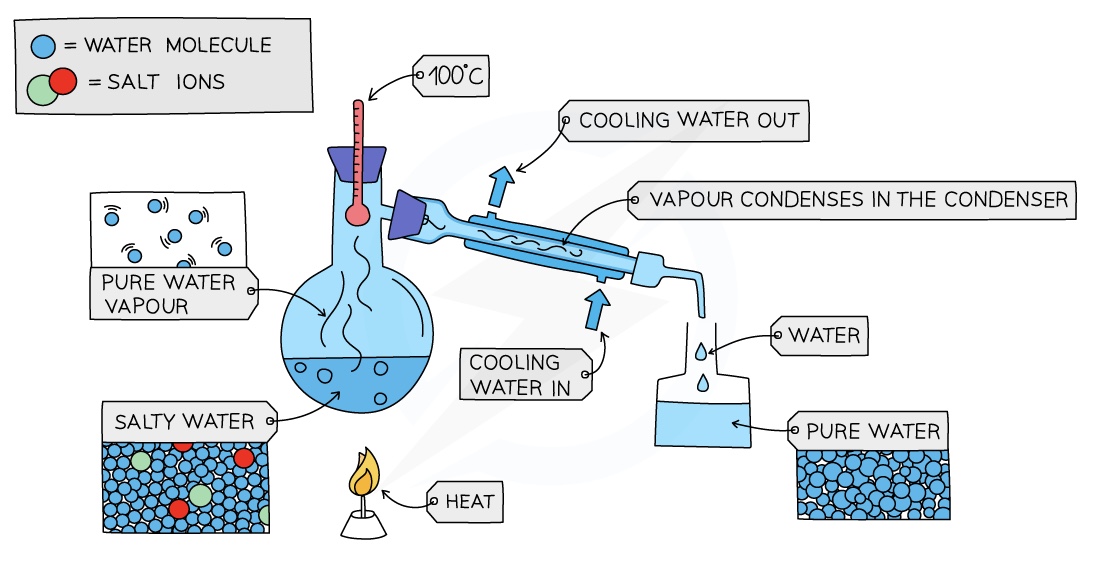
Simple Distillation
Used to separate a liquid and soluble solid from a solution (e.g. water from a solution of saltwater) or a pure liquid from a mixture of liquids
The solution is heated and pure water evaporates producing a vapour which rises through the neck of the round-bottomed flask
The vapour passes through the condenser, where it cools and condenses, turning into pure water which is collected in a beaker
After all the water is evaporated from the solution, only the solid solute will be left behind
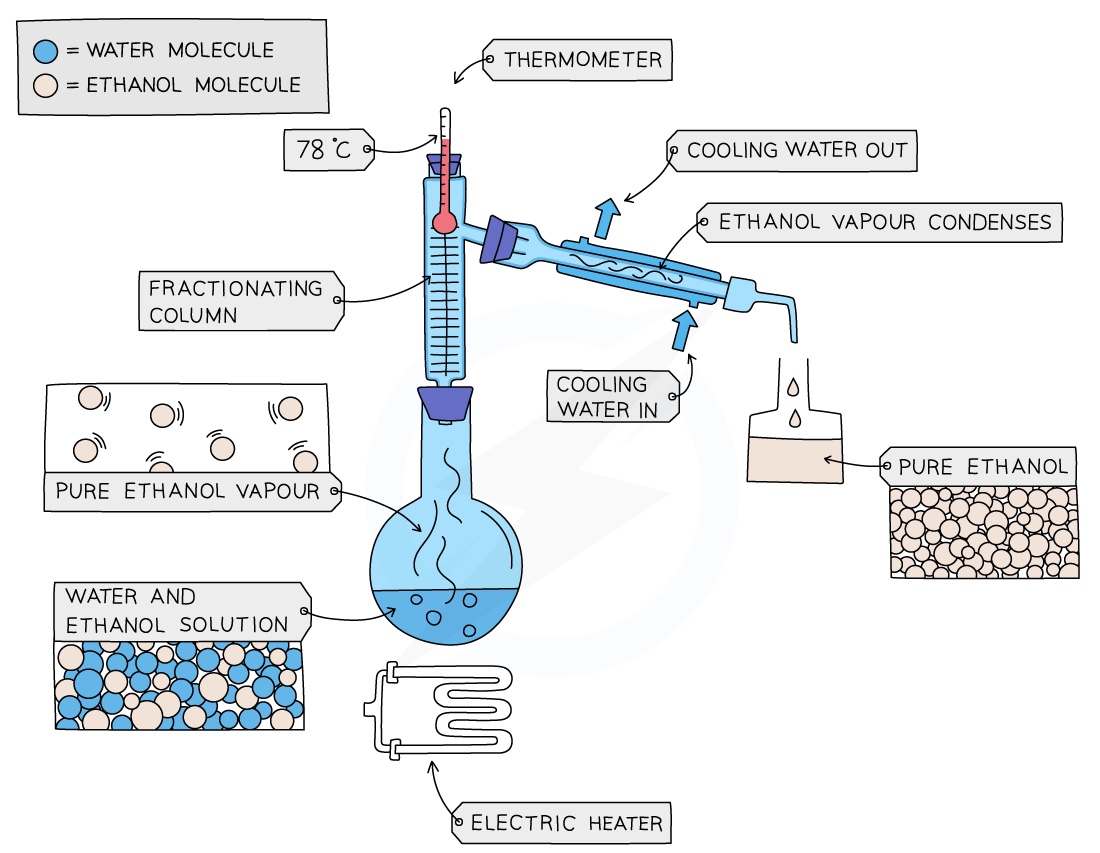
Fractional Distillation
Used to separate two or more liquids that are able to mix with one another (e.g. ethanol and water from a mixture of the two)
The solution is heated to the temperature of the substance with the lowest boiling point (ik it’s boring just keep reading)
This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker
All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture
For water and ethanol: ethanol has a boiling point of 78 ºC and water of 100 ºC. The mixture is heated until it reaches 78 ºC, at which point the ethanol boils and distils out of the mixture and condenses into the beaker
When the temperature starts to increase to 100 ºC heating should be stopped. Water and ethanol are now separated
Summary- for fractional distillation
- Mixture is heated
- Substances, due to their different boiling points, rise in different fractions
- A mixture of gases condense on the beads in the fractional column.
- The beads are heated to the boiling point of the lowest substance, so that substance being removed cannot condense on the beads.
- The other substances continue to condense and will drip back into the flask.
- The beaker can be changed after every fraction
Summary- for Simple distillation \n Used to separate a solvent from a solution
- Impure liquid is heated in a round bottom flask
- When it boils, the steam rises into the attached condenser
- Condenser cools the steams to a pure liquid and it drops into the beaker
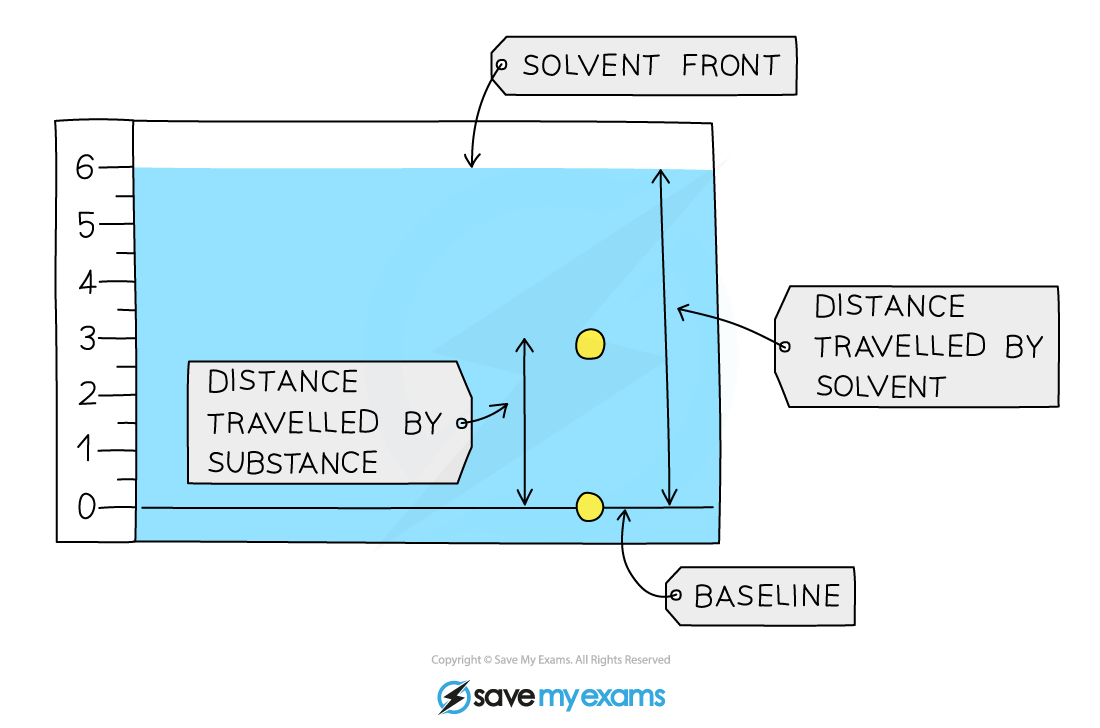
Retention Factor and RF a value
- These values are used to identify the components of mixtures
- The Rf value of a particular compound is always the same
- Calculating the Rf value allows chemists to identify unknown substances because it can be compared with Rfvalues of known substances under the same conditions
Calculation
Retention factor = distance moved by compound ÷ distance moved by solvent
The Rf value is a ratio and therefore has no units