CIE AS Biology 2.1: Biological Molecules
Molecular biology is closely linked with biochemistry. Metabolism is the sum of all biochemical reactions in the body.
Organic molecules always contain carbon and hydrogen. Oxygen and nitrogen are also commonly found in 99% of living organisms.
- carbon can form large chains or ring structures
- basic skeleton of organic molecules
Order of abundance: hydrogen > carbon > oxygen > nitrogen.
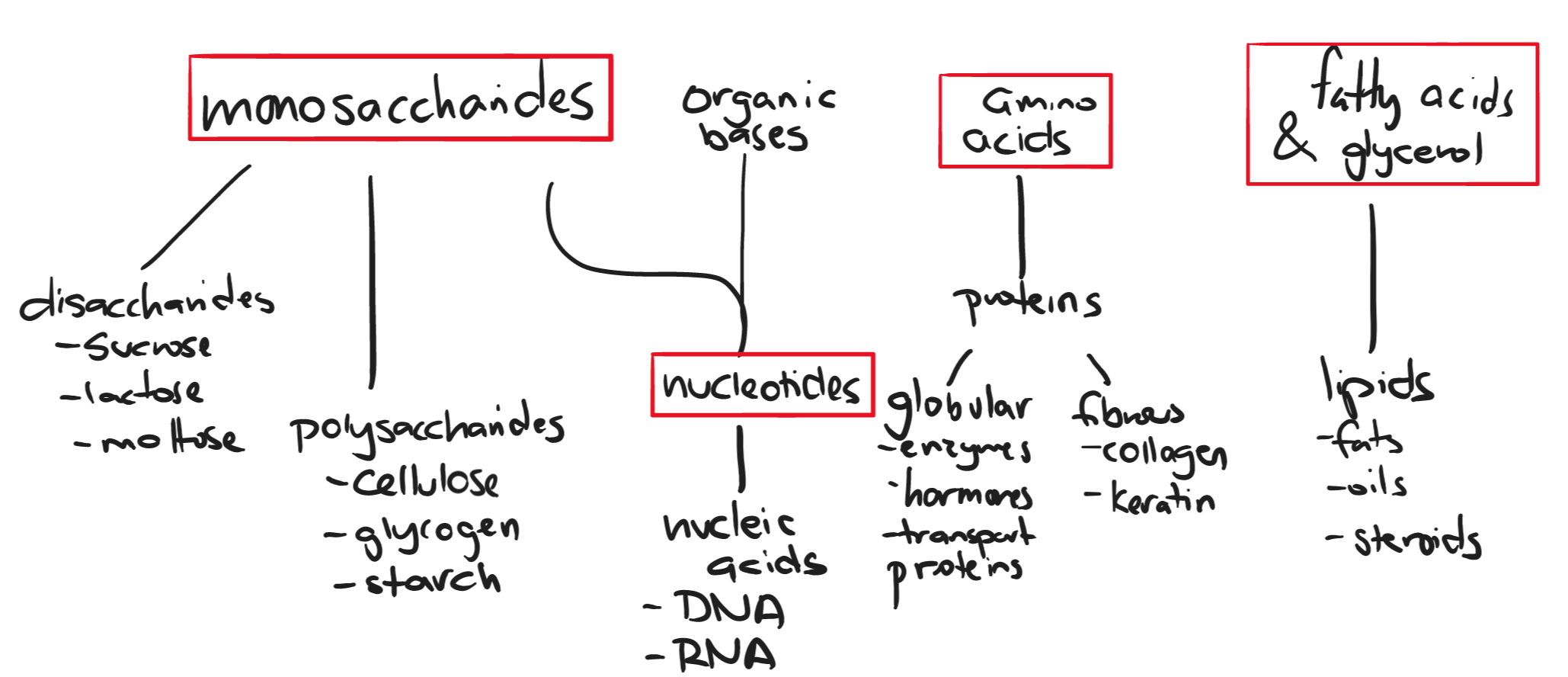
Monomers, Polymers, & Macromolecules
- monomer: single subunit e.g monosaccharides, nucleotides, amino acids, and fatty acids & glycerol
- polymer: made up of multiple monomers e.g polysaccharides, polynucleotides (nucleic acids), polypeptides (proteins)
- macromolecule: a polymer made up of large chains of monomers
 Carbohydrates
Carbohydrates
General formula: (CH2O)n
Carbohydrates are categorized depending on the number of carbon atoms they have. There are trioses (3C), pentoses (5C), and hexoses (6C).
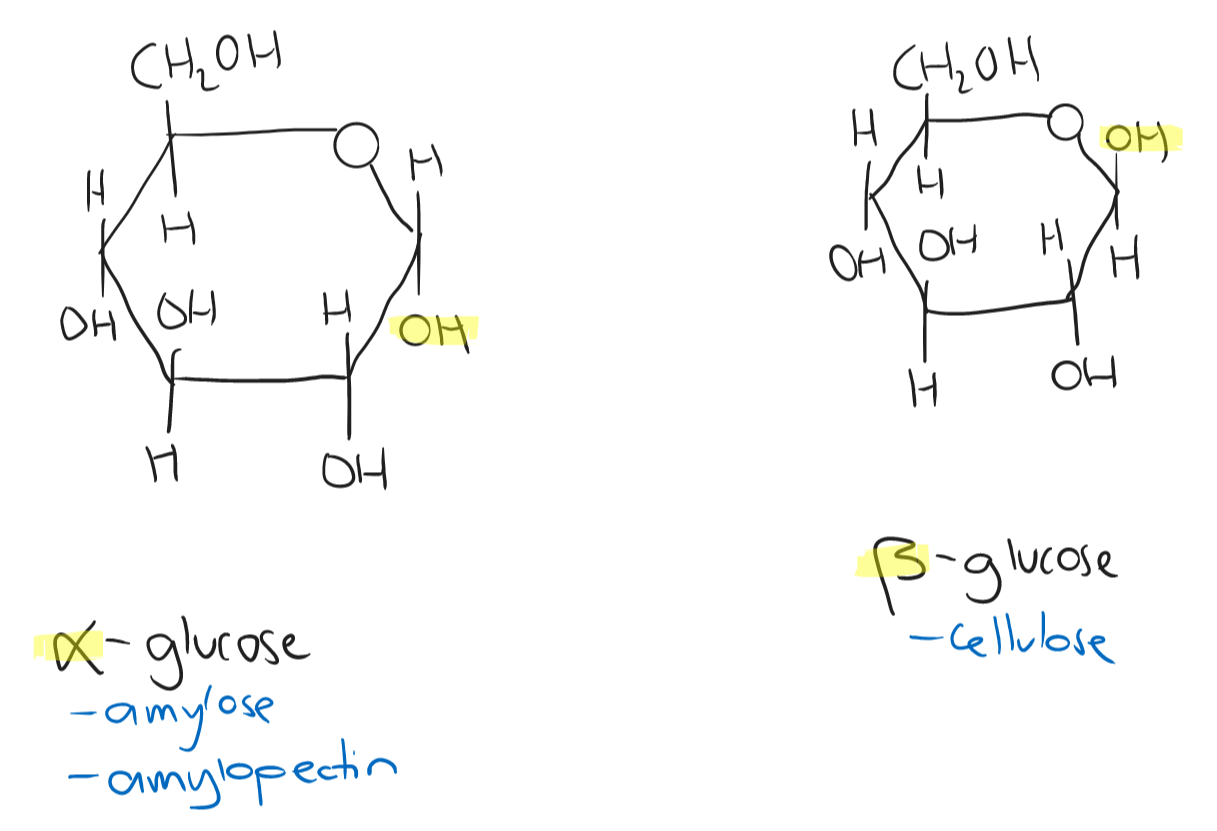 The bond formed between two monosaccharides is known as a glycosidic bond.
The bond formed between two monosaccharides is known as a glycosidic bond.
- A glycosidic bond occurs by a condensation reaction (removal of water) to form a disaccharide/polysaccharide.
- A glycosidic bond is broken by a hydrolysis reaction (addition of water) to produce separate monosaccharides.
| Starch | Glycogen | Cellulose | |
|---|---|---|---|
| Monomers | α-glucose; amylose and amylopectin | α-glucose; amylose and amylopectin | β-glucose |
| Bonding | amylose: 1,4 links \n amylopectin: 1,4 links and a branched 1,6 link | amylose: 1,4 links \n similar to amylopectin; 1,6 branched links | 1,4 linkage with every other β-glucose inverted; δ+ H atoms on hydroxyl (OH) groups form hydrogen bonds with δ- H atoms on other groups |
| Function | energy reserves in plants | energy reserves in animals found in the liver and muscle cells | strengthening material of cell walls |
Lipids
All lipids are organic molecules; non-polar and hydrophobic (insoluble) in water. They contain a lower proportion of oxygen compared to carbohydrates.
Lipids are made up of fatty acids and glycerol.
a fatty acid chain can be 15 to 17 carbon atoms long
unsaturated fatty acids contain carbon double bonds
- unsaturated fatty acids are more likely to melt more easily
- animal lipids are saturated (do not contain double bonds)
glycerol is an alcohol with three hydroxyl (-OH) groups
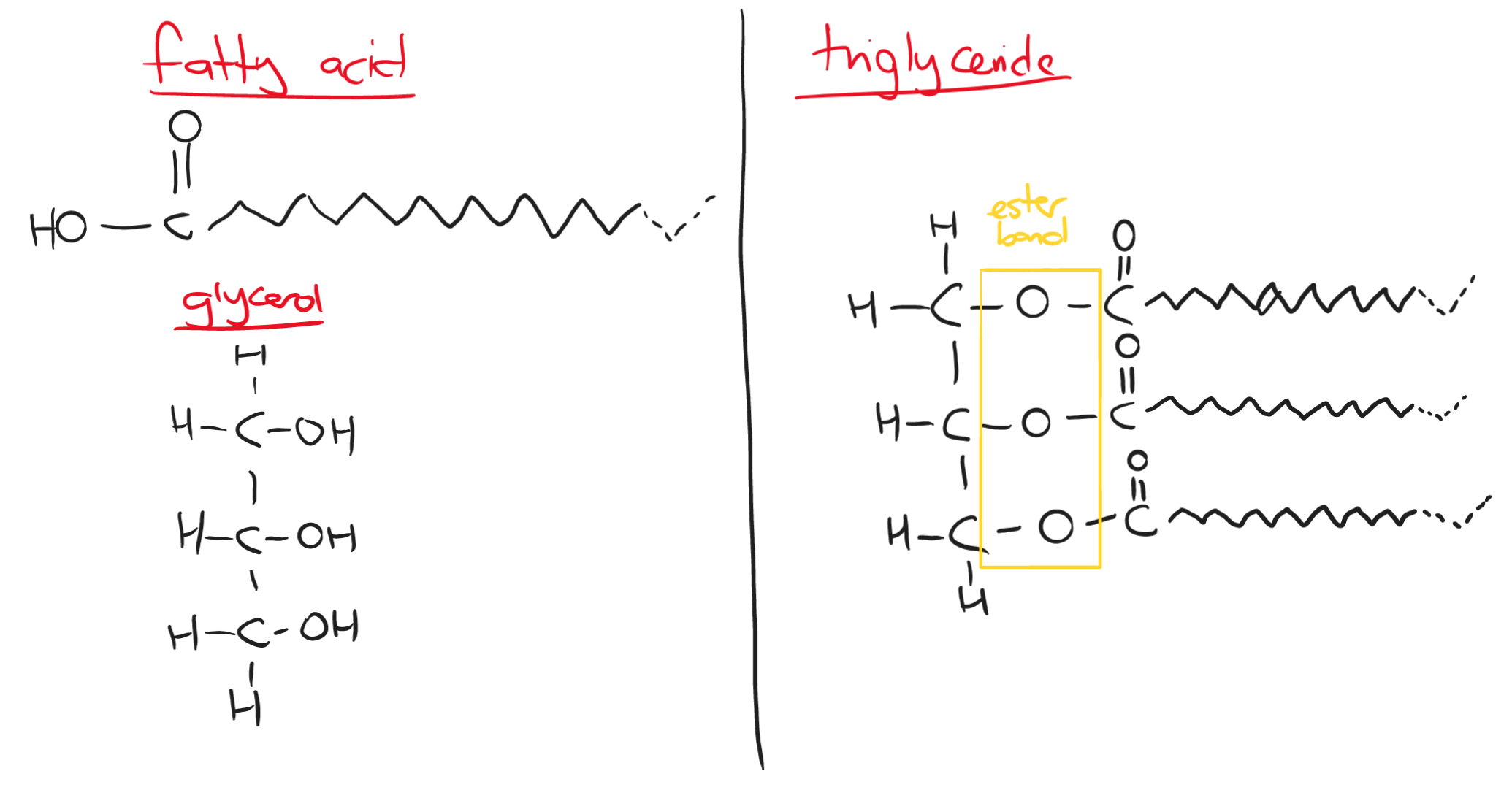 Triglycerides are the most common form of lipids. They are insoluble in water but soluble in other organic solvents (e.g ethanol).
Triglycerides are the most common form of lipids. They are insoluble in water but soluble in other organic solvents (e.g ethanol).The bond forming a triglyceride is an ester bond that is formed from a condensation reaction. It is broken apart by a hydrolysis reaction.
Lipids make excellent energy reserves because of the richness in carbon-hydrogen bonds
Fat is stored below the dermis (skin) and around the kidneys. Fat is responsible for;
- insulation
- metabolic source of water
- when oxidized, lipids produce carbon dioxide and water
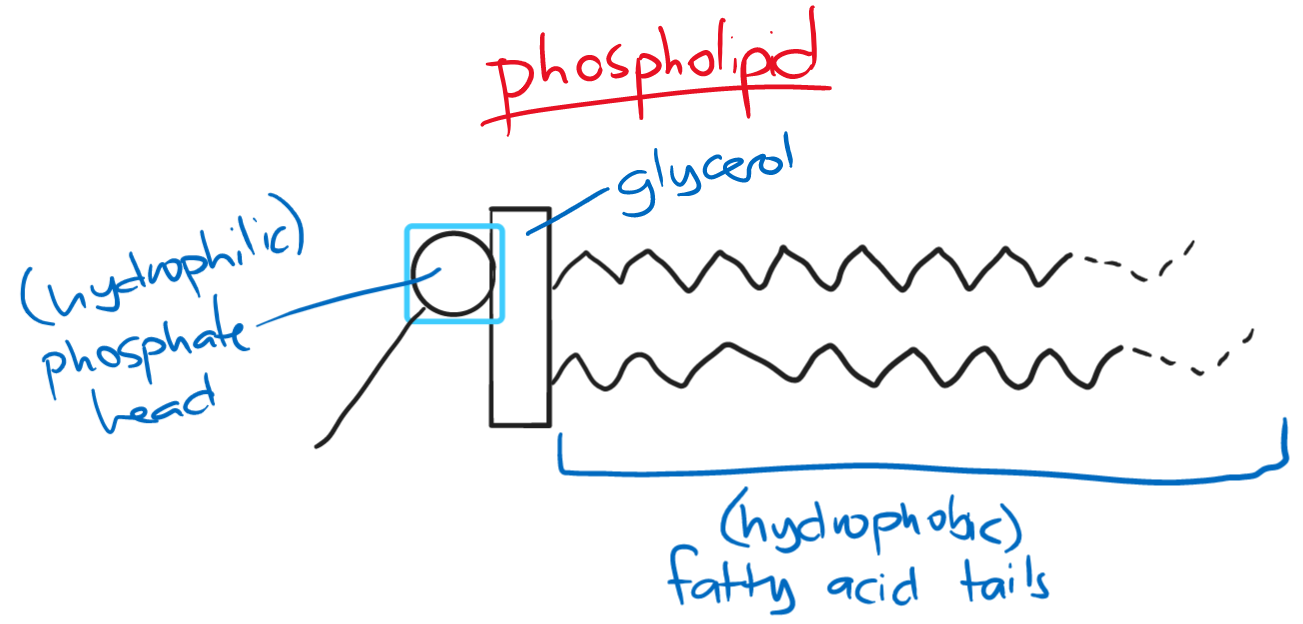
Phospholipids are similar to triglycerides. However, one fatty acid chain is replaced by a phosphate group.
- the phosphate head is hydrophilic; the fatty acid tails are hydrophobic
- the hydrophilic heads of phospholipids point towards water while the hydrophobic heads point away from water
- this forms an impermeable layer to hydrophilic substances
 Proteins
Proteins
Proteins are important in cells as they are responsible for:
- enzymes (amylase, trypsin, pepsin, lipase, etc.)
- cell membrane proteins (channel, carrier)
- hormones (insulin, estrogen, testosterone)
- immunoproteins (antigens)
- transport proteins (haemoglobin)
- structural proteins (keratin, collagen)
- contractile proteins (myosin)
There are 20 different amino acids found in all living organisms. These can be differentiated by their R group.
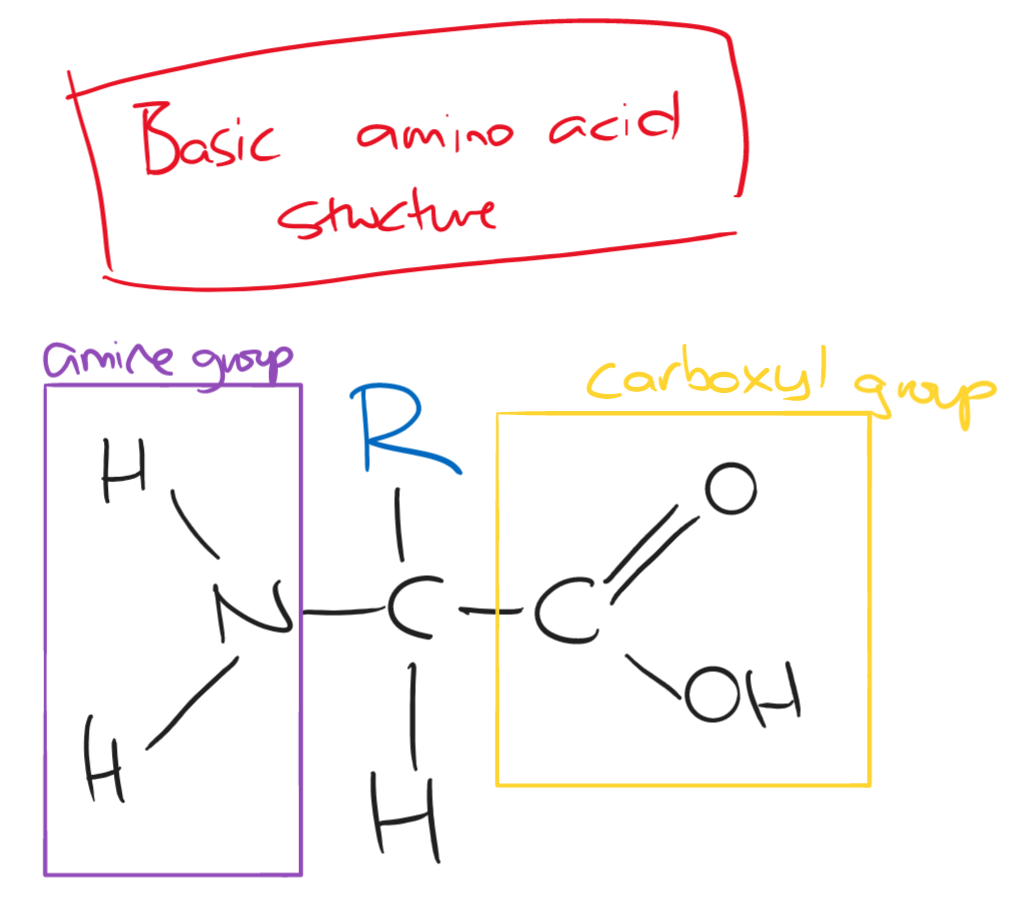 A dipeptide/polypeptide is made through a condensation reaction to form a peptide bond between two amino acids. It is broken down by a hydrolysis reaction.
A dipeptide/polypeptide is made through a condensation reaction to form a peptide bond between two amino acids. It is broken down by a hydrolysis reaction.
Proteins have four structures: primary (1°), secondary (2°), tertiary (3°), and quaternary (4°).
- Primary structure (1°)
- DNA of the cell determines primary structure due to the sequence of nucleotide bases that link specific amino acids in one chain
- a change in the sequence can change the protein’s function
- bonds present: phosphodiester bonds between nucleotides
- Secondary structure (2°)
- can form an α-helix or β-pleated sheet
- structure is common in fibrous proteins
- bonds present: hydrogen between amine and carboxyl groups
- Tertiary structure (3°)
- addition bonds between R groups (side chains) are made
- a compact structure is formed where the hydrophobic R groups are closer to the centre and the hydrophilic R groups are on the outside
- bonds present: hydrogen between amine, carboxyl, and R groups, disulfide, ionic
- hydrophobic interactions are present
- structure is common in globular proteins
- Quaternary structure (4°)
- multiple polypeptide chains join together to form one macromolecule
- bonds present are the same as in the tertiary structure
Some proteins can have prosthetic groups as part of their structure. These are non-proteins combined with proteins as it is required for their function.
There are globular and fibrous proteins:
| Features | Globular | Fibrous |
|---|---|---|
| Shape | roughly spherical/circular | long strands |
| Amino acid sequence | irregular; wide range of R groups used | repetitive; limited range of R groups used |
| Function | physiological \n metabolic | structural |
| Examples | haemoglobin, enzymes, insulin | collagen, keratin, myosin |
| Solubility | (generally) soluble in water | (generally) insoluble in water |
| Features | Haemoglobin | Collagen |
|---|---|---|
| Number of polypeptide chains | 4 (2 α-globin, 2 β-globin) | 3 (triple helix) |
| Shape | spherical/round | long, thin |
| Function | transport of oxygen; binds to haem group | in connective tissue e.g tendons, skin |
| Amino acid variation | variable | repetitive; every third amino acid is glycine |
| Prosthetic group? | present; haem group with iron (II) ion | not present |
| Solubility | soluble in water | insoluble in water |
Water
Water is dipole as it has a negative region (oxygen) and a positive (hydrogen) region. This makes water polar.
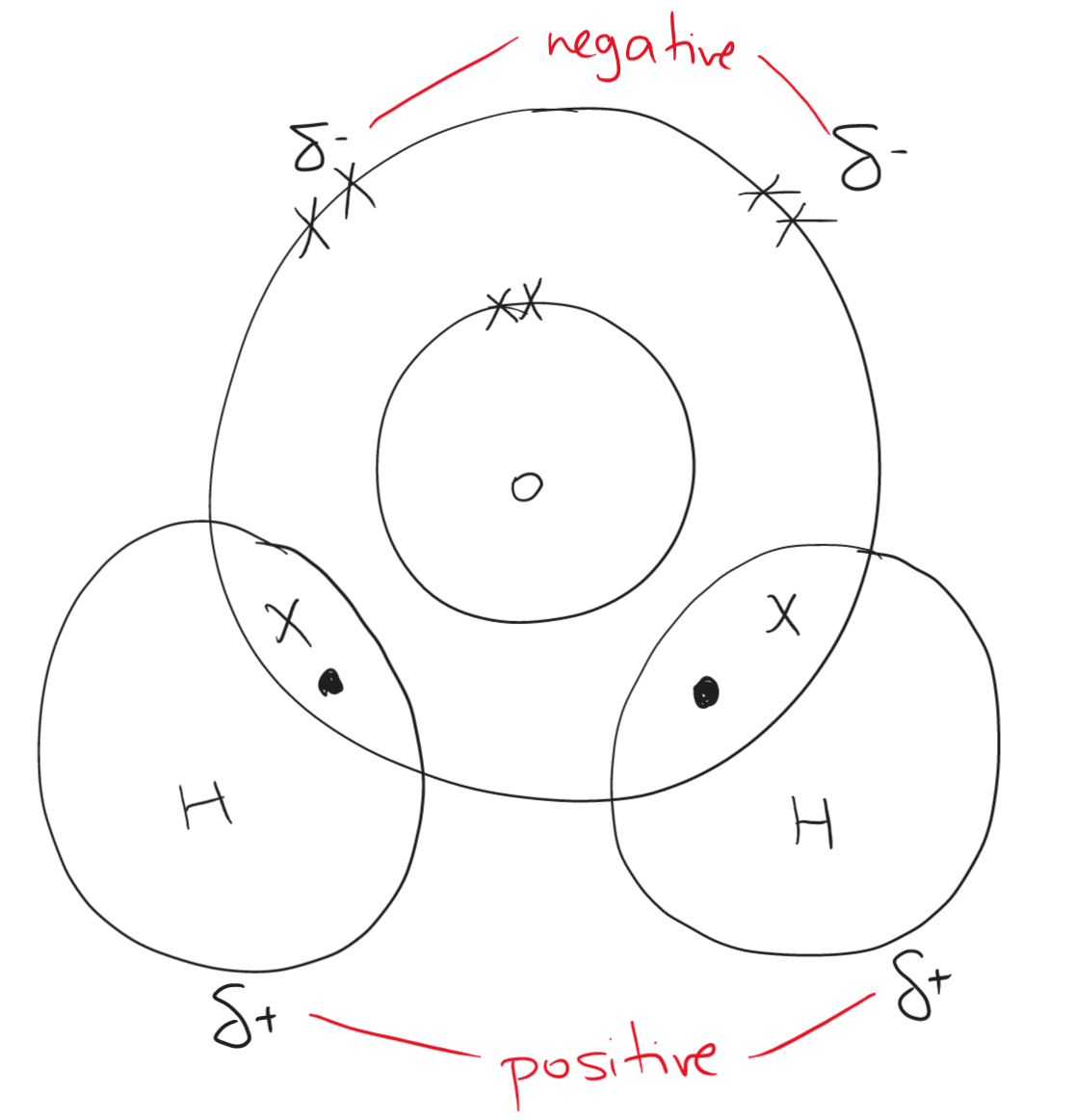 Properties of water include:
Properties of water include:
- solvent
- it is commonly used as a solvent for ions and other polar molecules due to their dipole regions (polarity)
- allows chemical reactions to occur
- all metabolic reactions in cells take place in water
- water is a transport medium in cells and organisms
- high specific heat capacity
- temperature is kept relatively constant
- more energy is required to raise the temperature of water
- this is due to the presence of hydrogen bonds
- high latent heat of vaporization
- to change water from a liquid to a gas, large amounts of thermal energy are needed
- this is due to the presence of hydrogen bonds
- water can, therefore, act as a coolant