Mass Spectrometer
The mass spectrometer can be used to determine all the isotopes present in a sample of an element and to therefore identify elements.
It needs to be under a vacuum otherwise air particles would ionise and register on the detector
The following are the essential 4 steps in a mass spectrometer.
1. Ionisation. The sample can be ionised in a number of ways. Two of these techniques are electron impact and electrospray ionisation.
Electron impact •A vaporised sample is injected at low pressure •An electron gun fires high energy electrons at the sample •This knocks out an outer electron •Forming positive ions with different charges e.g. Ti (g) Ti+ (g)+ e
Electron impact is used for elements and substances with low formula mass. Electron impact can cause larger organic molecules to fragment.
Electro spray Ionisation
The sample is dissolved in a volatile, polar solvent
injected through a fine needle giving a fine mist or aerosol
the tip of needle has high voltage
at the tip of the needle the sample molecule, M, gains a proton, H+ , from the solvent forming MH+ M(g) + H+ MH+ (g)
The solvent evaporates away while the MH+ ions move towards a negative plate
Electro spray ionisation is used preferably for larger organic molecules. The ‘softer’ conditions of this technique mean fragmentation does not occur.
2. Acceleration
Positive ions are accelerated by an electric field
•To a constant kinetic energy
KE=1/2nv2
KE = kinetic energy of particle (J) m = mass of the particle (kg) v = velocity of the particle (ms–1)
Given that all the particles have the same kinetic energy, the velocity of each particle depends on its mass. Lighter particles have a faster velocity, and heavier particles have a slower velocity.
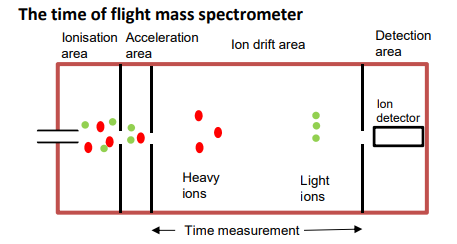
3. Flight Tube
•The positive ions with smaller m/z values will have the same kinetic energy as those with larger m/z and will move faster.
•The heavier particles take longer to move through the drift area.
•The ions are distinguished by different flight times
T=d/v t = time of flight (s) d = length of flight tube (m) v = velocity of the particle (m s–1 )
4. Detection
The ions reach the detector and generate a small current, which is fed to a computer for analysis. The current is produced by electrons transferring from the detector to the positive ions. The size of the current is proportional to the abundance of the species.
For each isotope the mass spectrometer can measure a m/z (mass/charge ratio) and an abundance
Sometimes two electrons may be removed from a particle forming a 2+ ion. 24Mg2+ with a 2+ charge would have a m/z of 12