Chemistry 11HL Structure 1-3
S.1
Classifying Matter
Mixtures
mixtures contain more than one element or compound in no fixed ratio
air is described as a mixture of gases because the seperate components are interspersed with each other but not chemically combined
nitrogen and oxygen have the same properties as they do in pure samples
homogenous mixture → uniform composition and properties
the inter-particle attraction within the different components must be similar in nature to those between the components in the mixture
heterogenous mixture → non-uniform composition and its properties are not the same throughout
interactions between components are different in nature
Solutions
Solute is dissolved in the solvent
Filtration
solid is seperated from a liquid or a gas using a membrane
solid is collected on the membrane as residue, filtrate containing solution passes through
Distillation
seperates solvent from solute
solvent has lower boiling point, so it is evaporated and collected as a gas then condensed
Paper Chromatography
small spots of solution containing the samples are placed on the baseline
paper is suspended to ensure it is saturated
different components have different water affinities so they seperate as the solvent moves up the paper
Kinetic Molecular Theory
states of matter are characterized by the different energies of the particles
temperature is directly related to the average kinetic energy of the particles
solid → gas = sublimation
gas → solid = deposition
b-c and d-e: energy is added to break intermolecular bonds, not increase kinetic energy as the average kinetic energy of the particles is sufficient enough already to leave
Celsius = Kelvin - 273.15, Kelvin = Celsius + 273.15
DIffusion
process by which particles of a substance spread out more evenly
occurs as a result of their random movements
particles with smaller mass diffuse more quickly
Ek=1/2 mv²
The Atom
Dalton’s model of the atom
all matter is composed of tiny indivisible particles called atoms
atoms cannot be created or destroyed
atoms of the same element are alike in every way
atoms of different elements are different
atoms can combine together in small numbers to form molecules
Electrons
negative charged particle
Protons
positively charged particle
Neutron
neutral charge, not positive or negative
Bohr model of the atom
Atomic/mass number
Atomic number: number of protons in the atom
no. of protons = no. of electrons if element has no overall charge
Mass number: number of protons + number of neutrons
electron mass is negligible, so this is regarded as the mass
Ions
positive ion - loses an electron - cation
negative ion - gains an electron - anion
Isotopes
different number of neutrons than the periodic table
same chemical properties
too many or too few neutrons causes radioactivity, making radioisotopes
Mass Spectra
determines the relative atomic masses of elements from their isotopic composition
mass spectrometer - measures mass and abundance of isotopes
amount of deflection is inversely proportional to mass/charge
fragmentation pattern provides insight on the structure of the compound
Relative atomic mass of an element
masses of all elements are in the range 10^-24 - 10^-22, so relative atomic mass is used
mass spectrum produced by the mass spectrometer using deflection patterns
Emission Spectra
different elements give out light of distinctive colours
electromagnetic radiation is emitted in different forms of differing energies
all travel at the same speed, but have differing wavelengths (v=λf)
c=λf (c=speed of light; 3×10^8) (λ=wavelength) (f=frequency)
an emission spectrum is produced when an atom moves from a higher to a lower energy level
when electromagnetic radiation passes through a collection of atoms, some of the radiation is absorbed and used to excite the atoms from a lower energy level to a higher energy level
spectrometer is used to analyze the transmitted radiation relative to the incident radiation and an absorption spectrum is produced
one packet of energy, a photon, is released for each electron transition
change in energy = energy of the photon emitted
energy of the photon = hf (h=Planck’s constant, f=frequency)
S2
S2.1: The Ionic Model
giving and taking of electrons to form electrostatic attraction
positive ion → cation, negative ion → anion
Transition Metals
When the ionization energies for the 1st, 2nd, 3rd, etc. are close to each other, the element will be able to lose those valence electrons easily
results in variable oxidation states depending on the number of valence electrons in the d and s shells
Compound Names
polyatomic ions
nitrate: NO3-
sulfate: SO4²-
phosphate: PO4³-
hydroxide: OH-
hydrocarbonate: HCO3-
carbonate: CO3²-
ammonium: NH4+
Ionic structures and properties
giant ionic lattice structures
made up of very strong electrostatic forces of attraction
ionic bonds or lattice enthalpy
physical properties depend on lattice structures
Lattice Enthalpy (LE)
energy needed to seperate into constituent ions (hence △H>0)
△H is a negative enthalpy change
LE values are a function of the ionic radii and charge
△H = (Knm)/(Rm+ + Rx-)
K - constant
n, m - magnitude of charges
Rm+, Rx- - ionic radii
increase in ionic charge = increase in ionic attraction between ions = increase in lattice enthalpy
increase in ionic radius of on of the ions = decreased attraction between ions = decreased lattice enthalpy
lattice enthalpy is greater for ions with a larger charge density as they have a small radius and are highly charged
Properties of ionic compounds
high melting + boiling point
due to high LE
generally soluble in water (polar) but not in non-polar liquids
good electrical conductivity in liquid/aqueous states
generally brittle
due to crystalline structure
low volatitilty
volatility - tendency of a substance to vaporize
Ionic character and electronegativity
ionic character is determined by the formula:
%ionic character = △Xp/3.2
△Xp=electronegativity difference
bonding continuum
Ionic → △Xp>1.8
Polar Covalent → 0<△Xp<1.8
Covalent → △Xp<1.8
S2.2: The Covalent Model
atoms sharing electrons
Octet Rule
tendency of atoms to gain a valence shell consisting of 8 electrons
pairs of electrons not involved in the bond are called lone pairs
ability of two atoms to form a covalent bond is due to similar strength with which they attract valence electrons
exceptions to the rule
applies to small atoms with less than 8 electrons
forms an incomplete octet
ex: BeCl2, BF3
Bond Strength
bond length → distance between 2 bonded nuclei
bond strength = bond enthalpy → energy required to break the bond
as bond length increases, bond enthalpy decreases
Coordination Bond
bond that is formed by both electrons in pair originating from the same atom
ex: H3O+
Valence Shell Electron Pair Repulsion (VSEPR)
because electron pairs in the same valence shell carry the same charge, they repel each other and so spread themselves as far as possible
electron pair = electron domain
all electron locations in valence shell, all single, double, triple = 1 electron domain
repulsion applies to electron domains (can be single/double/triple or non-bonding pairs)
total number of electron domains around central atom determines geometrical arrangement of electron domains
shape of molecules is determined by angles between bonded atoms
lone pairs and multiple bonds cause slightly more repulsion
lone pairs have a higher concentration of charge (not shared)
multiple bonds have a higher concentration of charge (more electrons)
non-bonding/lone pair > multiple bond > single bond
Structure
two electron domains
linear shape (bond angle of 180°)
three electron domains
triangular planar (bond angle of 120°) → all single bonds
bent/V-shaped (120°, 121°, 118°) → one double bond
bent/V-shaped (117° bond angle) → one double bond, one lone pair
four electron domains
tetrahedral (109.5°) → all single bonds
trigonal pyramid (107°) → one lone pair
bent/V-shaped (104.5°) → two lone pairs
Bond Polarity
polar bonds - differing electronegativities
different pulling strength for electrons
the more electronegative atom exerts a greater pulling power on the shared electrons → gains more “possesion”
bond dipole - two partially seperated opposite electric charges
the more electronegative atom becomes partially negative
the less electronegative atom becomes partially positive
increased electronegativity difference = increased bond polarity
pure covalent bonds → zero electronegativity difference
polar bonds introduce some ionic nature to the covalent bonds
Pure Covalent
equal sharing of electrons
discrete molecules
Polar Covalent
partial/unequal sharing/transfer of electrons
Ionic
complete transfer of electrons
lattice of oppositely charged ions
Molecular Polarity
depends on polar bonds it contains
depends on molecular geometry
non-polar
dipoles can cancel out, creating non-polar molecules
polar
if the molecule contains bonds of different polarity or the bonds are not symmetrically arranged, dipoles will not cancel out
creates a net dipole (turning force in electric field)
IR active
happens only when an overall dipole moment related to the position and vibration of its bonds is found
Covalent Network Structures
discrete molecules → finite amount of atoms
covalent networks → no finite number of atoms
single repeating pattern of covalent bonds
allotropes → different bonding/structural patterns of the same element in the same physical state, causing different chemical and physical properties
Allotropes of Carbon
Diamond
structure: sp3 hybridied and covalently bonded to 4 others tetrahedrally arranged in a regular repetitive pattern (angle → 109.5°)
non-conductor of electicity, all electrons are bonded
very efficient thermal conducter, better than metals
highly transparent, lustrous crystal
hardest natural substance, brittle, very high melting point
uses: polished for jewelry, tools and machinery for grinding/cutting glass
Graphite
structure: sp2 hybridized and covalently bonded to 3 others, forming hexagons in parallel angles with bond angles of 120° (weak London dispersion forces)
good electrical conductor; contains one delocalized electron per atom
not a good thermal conductor, unless the heat conducts parallel to crystal layers
non-lustrous, grey crystalline solid
soft and slippery due to sliding layers, brittle, very high melting point, stable mostly
uses: dry lubricant, pencils, electrode rods in electrolysis
Graphene
sp2 hybridized and covalently bonded to 3 others, forming hexagons with bond angles of 120° (single layer → 2D only) honeycomb/chicken wire
very good electrical conductor, one delocalized electron per atom
best thermal conductivity known
almost completely transperent
thickness of just one atom (2D) → thinnest material to ever exist, 100x stronger than steel (strongest), very flexible, very high melting point
uses: transmission electron microscopy (TEM) grids, photovoltaic cells, touchscreens, high performance electronic devices, etc.
Fullerene (C60)
sp2 hybridized, bonded in a sphere of 60 carbon atoms, consisting of 12 pentagons and 20 hexagons (closed spherical cage)
poor conductors of electricity, delocalized electron has little movement
very low thermal conductivity
black powder
very light and strong, reacts with potassium (K) to make superconducting crystalline material, low melting point
uses: lubricants, medical, industrial devices for binding specific target molecules; related forms are used to make nanotubes/nanobuds used as capacitators in the electronics industry, and catalysts
London Dispersion Forces
non-polar molecules do not have a permanent dipole
electrons behave somewhat like clouds of negative charge, density of the cloud could be greater over one atom at any moment
when there is a differing density, the bond will have seperation of charge, creating a weak dipole (temporary/instantaneous dipole)
creates weak forces of attraction that occur between opposite ends of two temporary dipoles
weakest form of intermolecular force
strength increases with molecular size (greater number of electrons)
LDF is the only force that exists for non-polar molecules
also exists in polar molecules, but is often overlooked for stronger forces
Dipole-dipole attraction
polar molecules have permanent seperation of charge (electronegativity difference)
known as a permanent dipole
opposite charges on neighbouring molecules attracting each other
strength varies on distance and relative orientation of the dipoles
Dipole-induced dipole attraction
occurs in mixtures with both polar and non-polar molecules
the permanent dipole from a polar molecule creates a temporary seperation of charge in the non-polar
act in addition to LDF (non-polar) and dipole-dipole attraction (polar)
van der Waal’s force: all 3 forces added together
Hydrogen Bonding
when a molecule contains hydrogen covalently bonded to fluorine, oxygen, or nitrogen (electronegative atoms)
particular case of dipole-dipole attraction
the large electronegativity difference between hydrogen and the bonded atoms results in the electron pair being pulled away from hydrogen
hydrogen now exerts a strong attractive force on a lone pair in the electronegative atom due to its small size and the absence of other electrons to shield the nucleus
strongest form of intermolecular force
Melting and Boiling Point
changing state = breaking intermolecular forces
covalent substances generally have lower MP and BP than ionic substances
relatively weak intermolecular forces < electrostatic attraction
covalent substances are generally liquid/gas at room temperature
strength of intermolecular forces increase with molecular size and extent of polarity
Solubility
non-polar substances are generally dissolvable in non-polar solvents by formation of LDF between solute and solvent
polar covalent compounds can generally dissolve in water (highly polar H2O) through dipole interactions and hydrogen bonding
solubility of polar compounds is reduced in larger molecules
polar bonds only a small part of the structure
non-polar parts reduce solubility
inability of non-polar groups to associate with water means non-polar substances do not dissolve well in water
polar substances have low solubility in non-polar solvents
they remain together due to dipole-dipole attractions
giant molecular are generally insoluble in all solvents
too much energy required to break the strong covalent bonds
Electrical Conductivity
covalent compounds do not contain ions, so they cannot conduct electricity in the solid or liquid state
some polar covalent molecules (when they can ionize) will conduct electricity
Resonance Structures
delocalization - tendency to be shared between more than bonding position
delocalized electrons spread out → greater stability for molecule/ion
delocalization occurs when there is more than one position for a double bond within a molecule
two equally valid positions for a double bond
expected is 1 single and 1 double bond, in reality is is 2 equal bonds, intermediate in length and strength
resonance - molecule is a combination of two Lewis formulas
electrons from the double bond delocalize and spread themselves equally between both possible bonding positions
shown with a dotted line
known as a resonance hybrid
resonance influences bond strengths/lengths which in turn can influence reactivity
Benzene (C6H6)
six carbon atoms arranged in a hexagonal ring, each bonded to a hydrogen atom in a triangular planar arrangement with bond angle 120°
true form of benzene is the resonance hybrid
circle represents equally spread delocalized electrons
1-1 ratio of carbon to hydrogen indicates a high degree of unsaturation, greater than that of alkenes or alkynes
does not show characteristic properties
no isomers, reluctant to undergo additional reactions
Expanded Octet
when the central atom is period 3 or lower, sometimes there are more than 8 electrons around the central atom
d orbitals available in the valence shell have energy values similar to those of the p orbitals
promotion of electrons (3p→3d) allows additional electron pairs to form
causes some elements to expand their octets (5-6 electron domains)
Species with five electron domains
triangular bipyramidal shape → angles of 90° and 120°
if one or more domains are non-bonding electrons, they will repel the most
one lone pair gives an unsymmetrical tetrahedron or see-saw shape (bond angles <120° and <90°)
to minimize additional repulsion and bonding domains, the lone pair must be located in an equatorial position (horizontal plane around central atom) instead of an axial osition (above/below horizontal plane)
two lone pairs give a T-shaped structure (bond angles <90°)
three lone pairs give a linear shape (bond angle 180°)
Species with six electron domains
octahedral shape with angles of 90°
no lone pairs of electrons → symmetrical octahedral shape
one lone pair → square pyramidal shape (bond angles slightly less than 90°)
two lone pairs → square planar shape (bond angles of 90°)
maximizes distance apart by arranging pairs on opposite sides
Formal Charge
formal charge used to predict a preferred Lewis formula
treats covalent bonds as if they were purely covalent with equal electron distribution
FC = V - (1/2 B + L)
V = valence, B = bonding, L = lone (number of electrons)
low FC means less charge transfer has taken place in forming a structure from its atoms
generally means most stable → preferred structure
sum of formal charges for a species must be equal to the charge
Sigma Bond
when two atomic orbitals combine head-on along the bond axis (imaginary line)
overlap of s, p, and hybrid orbitals in different combinations
always the bond that forms in a single covalent bond
electron density is concentrated between the nuclei of the bonded atoms
Pi Bond
when two p orbitals collide laterally (sideways-on)
electron density is concentrated above and below the plane of the bond axis
only forms within double and triple bonds
weaker than sigma bonds as electron density is further from nucleus
Sigma and Pi Bonds
s+s → sigma
s+p → sigma
p+p (head-on) → sigma
hybrid + s → sigma
hybrid + hybrid → sigma
p+p (laterally) → pi
single bond - 1 sigma
double bond - 1 sigma + 1 pi
triple bond - 1 sigma + 2 pi
Hybridization
formation of covalent bonds often starts with excitation of the atoms
amount of energy put in to achieve this is more than compensated by the extra energy released on forming bonds
if different orbitals are used in forming covalent bonds, unequal bonds are expected
instead, unequal atomic orbitals within an atom mix to form new hybrid atomic orbitals which are identical but different from the original bonds
hybrid orbirtals have different energies, shapes, and orientation in space from their parent orbitals
allows them to form stronger bonds by allowing for greater overlap
sp³ orbitals
1 s orbital and 2 p orbitals produce 4 sp³ orbitals
shape and energy have properties of s and p, but more like p than s
sp² orbitals
1 s orbital and 2p orbitals produce 3 sp² orbitals
sp orbitals
1 s orbital and 1 p orbital produce 2 sp orbitals
Carbon → Hybridization
C: atomic number = 6 (1s²2s²2px12py1)
forms 4 covalent bonds, but only has two singly occupied bonding electrons
excitation occurs (2s → 2p) to change from ground state
sp³ hybridization
orbitals orientate themselves at 109.5°, forming a tetrahedron
each hybrid orbital overlaps with an atomic orbital → 4 sigma bonds
sp² hybridization
when carbon forms a double bond
orientate themselves at 120°, forming a triangular planar
each hybrid orbital overlaps with a neigbouring atomic orbital → 3 sigma bonds
as the 2 carbon atoms approach each other, the p orbitals in each atom that did not hybridize overlap sideways
forms a pi bond
double bond (C2) → 1 sigma, 1 pi
characteristic lobes of electron density above and below the bond axis
sp hybridization
orientate themselves at 180°, giving a linear shape
overlap of the two hybrid orbitals with other atomic orbitals → 2 sigma bonds
when carbon forms a triple bond
C2H2
each carbon atom has 2 unhybridized p orbitals that are orientated 90° to each other
combines to form 2 pi bonds
four lobes of electron density turns into a cylinder of negative charge around the atom, making the molecule susceptible to electrophilic reactants (attracted to electron-dense regions)
Hybridization and Molecular Geometry
tetrahedral → sp³
triangular planar → sp²
linear → sp
lone pairs can also be used in hybridization
non-bonding pairs can also hybridize
ex: NH3 → lone pair in N resides in the sp³ orbital
Hybridization and Benzene (C6H6)
each of the 6 carbon atoms are sp² hybridized
forms 3 sigma bonds (120°) → planar shape
leaves the unhybridized p electron on each carbon atom
dumbbell shape perpendicular to the plane of the ring
do not form pi bonds but effectively overlap in both directions
spreads themselves evenly to be shared by all 6 carbon atoms
forms a delocalized pi electron cloud
electron density is concentrated in 2 donut-shaped rings above and below the plane
2.3: The Metallic Model
Metallic Bonding
metals: low ionization energies so they react by losing valence electrons forming a positive ion
metallic character: loss of control over outer shell electrons
when there is no other element present to accept the electrons and form an ionic compound, the outer electrons are held loosely by the nucleus so they ‘wander off’
delocalized electrons
metal atoms form a regular lattice structure through which electrons move freely
metallic bonding: force of electrostatic attraction between lattice of cations and delocalized electrons
Uses of metals
Good electrical conductivity
because of highly mobile delocalized electrons
used for electrical circuits (copper)
Good thermal conductivity
because of delocalized electrons and closely packed ions
used for pots and pans for cooking
Malleable (can be shaped under pressure)
because of the lack of direction in the movement of delocalized electrons
used for machinery
Ductile (can be drawn out into threads)
because the metallic bond remaining intact while formation changes
used for electric wires and cables
High melting points
because of strong electrostatic forces
used for high-speed tools
Shiny, lustrous appearance
because delocalized electrons in metal crystal structure reflect light
used for jewelry
non-directional nature of metallic bonding allows metals to mix with other metals or non-metals in the molten state
resulting mixture is an alloy
enhances properties of the metallic structure
Metallic bond strength
determined by
number of delocalized electrons
charge of the cation
radius of the cation
the greater the electron density and the smaller the cation, the greater the electrostatic attraction
Across a period
increasing melting point
greater attraction between ions and delocalized electrons
lower degree of reactivity
Down a group
decreasing melting point
weaker attraction between ions and delocalized electrons
higher degree of reactivity
Transition elements
elements with an incomplete d-sublevel OR elements that can give rise to cations with an incomplete d-sublevel
proximity in energy between outer occupied sublevels enables them to delocalize large amounts of d-electrons to form metallic bonds
Transition elements: High melting point
metals have a large amount of delocalized electrons and a large positive charge on the metal cations which leads to strong metallic bonding → high melting points
transition metal trends are less evident due to ability of transition elements to delocalize large numbers of electrons and the similar ionic radii
difficult to predict trends accurately compared to the s-block metals
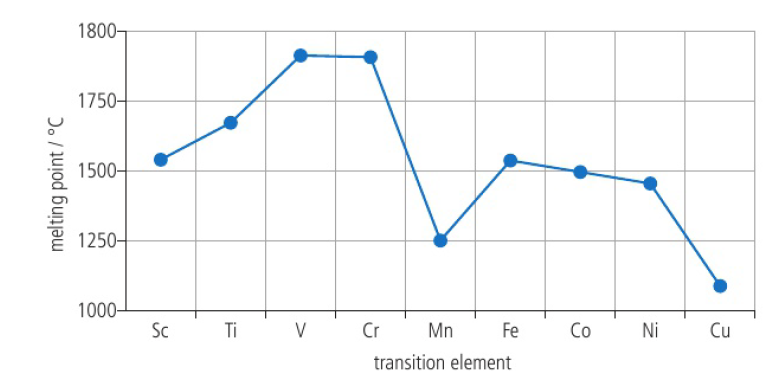
Transition elements: High electrical conductivity
metals have a large amount of delocalized electrons which increases their conductivity
example: copper (Cu) is used in wires
2.4: Models to Materials
Bonding Triangle
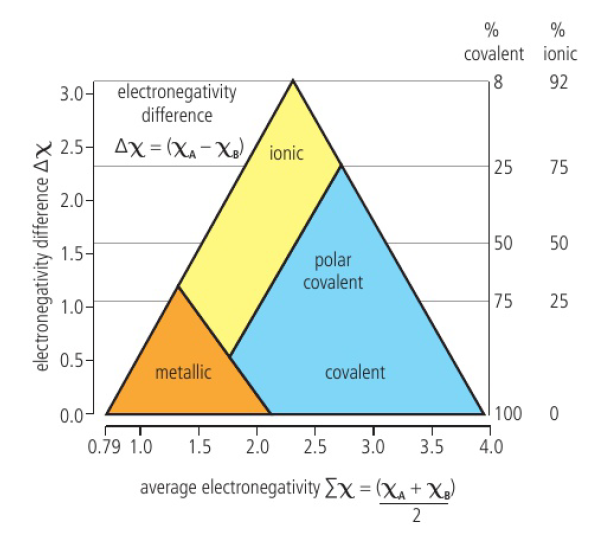
bonding seen as a continuum (ionic, covalent, metallic) → different bonding types are present to different degrees
position on triangle determined from electronegativity
high electronegativity difference = ionic
low electronegativity difference = covalent or metallic
intermediate electronegativity difference = polar covalent
Composite Materials
mixture between two or more different materials
materials have seperate phases (different positions on bonding triangle)
mixture retains properties of individual materials that compose it
example: fibreglass, concrete
Alloys
produced by adding one metal element to another metal (or carbon) in liquid/molten state so the different atoms can mix
in solid, ions of the different metals are scattered through the lattice
forms a structure of uniform composition
metallic bonds are present → delocalized electrons bind the lattice
possible due to the non-directional nature of the delocalized electrons and accomodation of the lattice to different sizes of ions
alloys have properties distinct of component elements (different packing of cations in lattice)
pure metal → regular arrangement of atoms
interrupted in an alloy by different cations
more difficult for atoms to ‘slip over each other’ → stronger
alloy is stronger, more chemically stable, and more resistant to corrosion
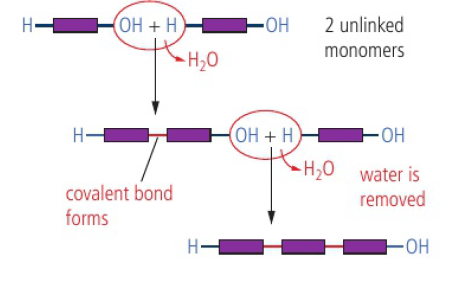
Polymers
monomers (small molecules) are able to react together to form a linked chain held together by covalent bonds, forming a polymer
polymers are macromolecules → composed of thousands of atoms and so are relatively large compared with other molecules
nature/properties of a polymer vary with the monomer, length, and amount of branching
structure is shown as a repeating unit with open bonds on each end
natural polymers - found naturally (example: protein, starch, DNA)
synthetic polymers - human-made and non-biodegradable (example: plastics)
Addition Polymers
addition reaction - a multiple bond in a molecule breaks and creates new bonding positions
alkenes/alkynes have double/triple carbon-carbon bonds respectively so they readily undergo addition reactions
they can act as monomers and form addition polymers
%atom economy = molar mass of desired product / molar mass of all reactants x 100
addition polymerization reactions do not generate a by-product so it has a 100% atom economy
Condensation Polymers
condensation reaction - two functional groups react to form a new covalent bond with the release of a small molecule (H2O, HCl, NH3, etc.)
A-OH + H-B → A-B + H2O
to form condensation polymers, monomers must have functional groups (active ends)
allows them to form new covalent bonds with neighbours on both sides
the functional groups in neighbouring molecules must be able to react together
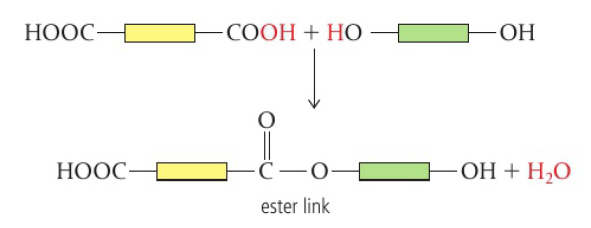
Carboxylic acid + alcohol → polyester (ester link)
when one monomer has two carboxylic acid groups (COOH) and the other has two alcohol groups (OH), an ester link forms between them
chain extends in both directions → polyester
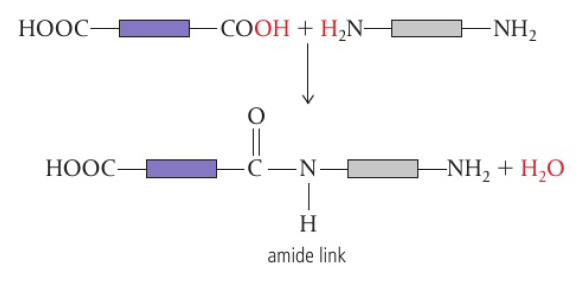
Carboxylic acid + amine → polyamide (amide link)
when one monomer has two carboxylic acid groups (COOH) and another monomer has two amine groups (NH2), an amide link forms between them
forms a polymer known as polyamide
S3
3.1: Classification of Elements
(see 3.2 Periodic Trends old syllabus for more detail)
Atomic Radius
increases down a group and decreases across a period
down a group: number of occupied electron levels increase
across a period: number of occupied electron levels stay the same but the number of protons increase, increasing the nucleus’ force of attraction to the outer electrons
Ionic Radius
positive ions are larger than parent atoms due to loss of outer energy level (valence)
negative ions are smaller than parent atoms due to addition of electrons
increased electron repulsion causes electrons to move
increase in nuclear charge (number of protons) causes ionic radius to decrease
increased attraction between outer electrons and nucleus
ionic radius increases down a group due to increased amount of occupied energy levels
Ionization Energy
increases across a period and decreases down a group
Electron Affinity
decreases down a group and increases across a period
Electronegativity
decreases down a group and increases across a period
across → increase in nuclear charge increases attraction between nucleus and bond electrons
down → increases distance between nucleus and bond electrons so reduced attraction
Group 1: Alkali metals
physical properties
good conductors of electricity and heat (mobility of outer electrons)
low density
shiny grey surfaces when freshly cut with a knife
chemical properties
very reactive metals
forms ionic compounds with non-metals
forms single charged ions (X+)
reactivity increases down group (lower IE)
reaction with water: forms hydrogen and metal hydroxide
lithium: floats and reacts slowly (releases hydrogen but keeps shape)
sodium: reacts vigorously (heat produced melts the unreacted metal)
potassium: reacts more vigorously (heat produced ignites hydrogen)
Group 17: Halogens
physical properties
coloured
gradual change from gases (F2, Cl2) to liquid (Br) and solid (I2, At2)
chemical properties
very reactive non-metals
reactivity decreases down group (lower attraction)
form ionic compounds with metals and covalent compounds with non-metals
displacement reactions
the more reactive halogen displaces the less reactive halogen
halides
halogens produce insoluble salts with silver forming precipitates
Period 3 Oxides
ionic character of period 3 oxides decrease from left to right
electronegativity value approaches oxygen, so the difference is less
ionic oxides
dissolve in water to form alkaline solutions
reacts with acid to form a salt and water
non-metallic oxides
reacts with water to form acidic solutions
amphoteric oxides
essentially insoluble (does not affect pH when added to water)
shows both basic and acidic behaviour
Acid Rain
produced by non-metal oxides
sulfur oxides
S(s) + O2(g) → SO2(g) sulfur dioxide
H2O(l) + SO2(g) → H2SO3(aq) dissolve in rainwater
2SO2(g) + O2(g) → 2SO3(g) sulfur trioxide
H2O(l) + SO3(g) → H2SO4(aq) dissolve in rainwater (acid)
nitrogen oxides
N2(g) + O2(g) → 2NO(g) nitrogen monoxide
N2(g) + 2O2(g) → 2NO2(g) and 2NO(g) + O2(g) → 2NO2(g) nitrogen dioxide
H2O(l) + 2NO(g) → HNO2(aq) + HNO3(aq) dissolve in rainwater
2H2O(l) + 4NO2(g) + O2(g) → 4HNO3(aq) oxidized
Oxidiation States
oxidation is:
addition of oxygen
removal of hydrogen
electron loss
an increase in oxidation state
rules to assign oxidation states:
atoms in the free (uncombined) element have an oxidation state of zero
in simple ions, the oxidation state is the same as charge of the ion
oxidation states of all atoms in a neutral compound must add up to zero
oxidation states of all atoms in a polyatomic ion must add up to the charge
usual oxidation state for an element in a compound is the one most commonly found
F (fluorine) has oxidation state of -1 all the time (most electronegative)
O (oxygen) has oxidation state of +2 except in peroxides
Cl (chlorine) has oxidation state of -1 except when bonded to more electronegative ions
H (hydrogen) has oxidation state of +1 except when forming ionic hydrides
oxidation state of a transition metal in a complex ion can be found using the charge on the ligands
Transition Metals
metals in the d-block have similar physical and chemical properties
zinc is not a transition metal
has a full d sublevel in both species
physical properties
high electrical and thermal conductivity
high melting point
high tensile strength
malleable and ductile
chemical properties
forms compounds with more than one oxidation state
form a variety of complex ions
form coloured compounds
acts as catalysts when either elements or compounds
magnetic properties
only found in iron, nickel, and cobalt
due to presence of unpaired electron
every spinning electron can act as a magnet
Variable Oxidation States
transition metals display a wide range of oxidation states
all transition metals show both +2 and +3 oxidation states
maximum oxidation states increase in steps of +1 and reaches a maximum at manganese then decreases in steps of -1
oxidation states above +3 generally show covalent character
compounds with higher oxidation states tend to be oxidizing agents
Coloured Compounds
transition metal ions in solution have a high charge density
attracts water molecules which form coordination bonds with the positive ions
complex ions are formed when a central ion is surrounded by molecules/ions that possess at least one lone pair of electrons (ligands)
number of coordination bonds from the ligands to the central ion is called the coordination number
colours appear because of a spurt in the d-orbital’s energy levels
when light passes through, it excites electrons and increases the energy level for these electrons
the ions absorb some colours and reflects the ones opposite it
when the energy (light) is absorbed, the d-orbitals split into two levels
3.2 Functional Groups: Classification of Organic Compounds
empirical formula → simplest whole-number ratio of atoms
molecular formula → actual number of atoms
full structural formula → shows every bond and atom at 90°/180°
condensed structural formula → omits bonds, displays minimal information
skeletal formula → shorthand representation of a structural formula
aromatic compounds: molecules which contain a benzene ring
catenation: ability of carbon to link to itself and form chains/rings
Functional Groups
atoms/groups of atoms that are present in organic compounds and are responsible for a compound’s physical properties and chemical reactivity
*halogen atoms are regarded as substituents as they have taken the position of a hydrogen atom
IUPAC names example: chloroethane, 2-bromopropane, etc.
**syllabus does not require knowledge of arenes as compounds but expects you to recognize the phenyl group when it is present in a structure
naming is also not required
Functional Groups: chemical reactivity
reaction pathway → several reactions to produce a target compound
product of one reaction is reactant of the next
ex: ethane (CH2) → ethanoic acid (CH3COOH)
Amino acids: condensation reaction to form link
amino acids contain 2 functional groups:
amine (-NH2) and carboxylic acid (-COOH)
amino acids react together via condensation reaction
molecule of water eliminated, acid and amino groups form new bond
bond is a substituted amide link (peptide bond) forming a dipeptide
the dipeptide still has a functional group (-NH2, -COOH)
can perform a condensation reaction again, forming a tripeptide and eventually a chain of many linked amino acids (polypeptide)
Homologous Series
organic compounds are classified into ‘families’ of compounds
successive members of a homologous series always differ by CH2
ex: C2H6, C3H8, C4H10 → alkanes
members of a homologous series can be represented by the same general formula
members of a homologous series show a trend in physical properties
because they differ by CH2, carbon chains get progressively longer
so higher B.P. for example
longer chians = increased London dispersion forces
Functional groups: physical properties
most volatile → least volatile
alkane > halogenoalkane > aldehyde > ketone > alcohol > carboxylic acid
London dispersion forces → dipole-dipole interaction → hydrogen bonding
increasing strength of intermolecular attraction →
increasing boiling point →
chain length and functional groups affect intermolecular forces
polar functional groups = dipole-dopole or hydrogen bonding
IUPAC Naming
Identify the longest straight chian of carbon atoms
1=meth-, 2=eth-, 3=prop-, 4=but-, 5=pent-, 6=hex-, etc.
Identify the functional group
numbered → nuimber has to be the smallest value possible
Identify the side chains or substituent groups
halogenoalkane (-F, -Cl, -Br, -I), amine (-NH2)
Esters and Ethers
esters → form when the alkyl group of an alcohol replaces the hydrogen of a carboxylic acid in a condensation reaction
R-COOH + R’OH → R-COO-R’ + H2O
the stem comes from the parent acid but the alkyl group of the alcohol is the prefix
ex: ethanol + ethanoic acid → ethylethanoate
ethers → 2 alkyl chains linked by an oxygen atom
R-O-R’
the longer chain will be the stem and retains its alkane name
the shorter chain is regarded as a substituent and is given the prefix alkoxy
ex: methoxypropane, ethoxyethane
prefix - stem - suffix
prefix - position, number, and name of substituents
stem - number of carbon atoms in longest chain
suffix - class of compound determined by functional group
Structural Isomers
same molecular formula but different arrangements of the atoms
each isomer is a distinct compound
unique physical and chemical properties
the more branching that is present in an isomer, the lower its boiling point
reduced surface contact weakens London dispersion forces
primary carbon → attached to functional group and at least 2 hydrogen atoms
seconday carbon → attached to functional group, one hydrogen atom, and 2 alkyl groups
tertiary carbon → attached to functional group and 3 alkyl groups
Stereoisomers
atoms are attached in the same order but differing in spatial or 3D arrangements → requires 3D representation
Isomerism - structural + stereo
Stereo - configurational + conformational
Configurational - cis-trans + optical
Conformational Isomers (not needed)
spontaneously interconnect through bond rotations and so cannot be isolated seperately (usually)
some conformers of a compound may be more stable than others so are favoured → influences reactivity of the compound
Configurational isomers
permanent difference in geometry
cannot be interconverted and exist as seperate compounds with some distinct properties
Cis-trans isomers
double-bonded molecules
consists of one sigma and one pi bond (pi bond forming by sideways overlap of two p orbitals)
free rotation around this double bond is not possible
would push p orbitals out of position and pi bond breaks
when the molecule contains two or more different groups attached to the double bond, these can be arranged to give 2 different isomers
cis → same side, trans → opposite side s
cyclic molecules
cycloalkanes contain a ring of carbon atoms that restricts rotation
bond angles are strained from the tetrahedral angles in parent alkane
Optical isomers
chiral - carbon atom attaches to 4 different atoms/groups
also known as asymmetric or stereocentre
the four groups arranged tetrahedrally with bond angles of 109.5° can be arranged in 2 different 3D configurations which are mirror images
known as enantiomers → chiral molecules
have opposite configurations at each chiral center
diastereoisomers → have opposite configurations at one or more (but not all) chiral centers
not mirror images of each other
Properties of enantiomers
optical activity → interaction with light
when a beam of plane-polarized light passes through a solution of optical isomers, they rotate the plane of polarization
optically active → seperate solutions of enantiomers (at the same concentration) rotate plane-polarized light in equal amounts but opposite directions
racemic mixture → chiral compound with equal concentration of 2 optical isomers
two optical isomers’ rotations cancel out, so racemic mixtures are optically inactive
reactivity with other chiral molecules
when a racemic mixture is reacted with a single enantiomer of another chiral compound, the two components of the mixture (+ and -) react to produce different products
products have distinct chemical and physical properties so can be seperated
resolution → two enantiomers seperated from a racemic mixture
Mass Spectrometry
used to find mass of individual atoms and finding relative abundances of different isotopes → also finds relative molecular mass of a compound
Fragmentation patterns
ionization process → shooting an electron from electron gun then hitting the incident species and removing an electron
X(g) + e- → X+(g) + 2e-
X is a molecule
collision can be so energetic the molecule breaks into different fragments
fragmentation pattern is used as evidence to find the structure of a compound
peak (largest mass/charge) is molecular ion that passed without fragmenting
Infrared Spectroscopy
frequency of radiation is often measured as number of waves per centimeter (wavenumber)
radio waves can be absorbed by certain nuclei, reversing their nuclear spin (environment)
used in nuclear magnetic resonance (NMR)
microwaves cause molecules to increase their rotational energy (bond lengths)
infrared radiation is absorbed by certain bonds causing them to stretch/bend (bonds)
visible/ultraviolet light can produce electronic transitions (electronic energy levels)
x-rays are produced when electrons make transitions between inner energy levels
produce diffraction patterns (molecular/crystal structure)
Natural frequency of a chemical bond
chemical bonds are like springs/rulers
each bond vibrates and bends at a natural frequency
depends on strengths and atom masses
light atoms vibrate at higher frequencies (less weight)
multiple (stronger) bonds vibrate at higher frequencies
simple diatomic molecules can only vibrate when the bond stretches
Exciting bonds
energy needed to excite bonds occur in the infrared (IR) region
only polar diatomic molecule bonds will interact with IR radiation
presence of positive and negative charge allows the electric field component of the IR radiation to excite the vibrational energy
change in vibrational energy produces change in dipole moment
intensity of absorption depends on polarity
Stretching and Bending
in a polyatomic molecule (like water), it is more correct to consider the molecule stretching and bending as a whole, rather than considering individual bonds
ex: water can vibrate at 3 fundamental frequencies
symmetric stretch, asymmetric stretch, symmetric bend
each of the modes of vibration results in a change of dipole in the molecule
can be detected with IR spectroscopy
for a symmetrical linear molecule (like carbon dioxide), there are 4 modes of vibration
symmetric stretch is IR inactive: no change in dipole moment
dipoles of both C=O bonds are equal and opposite throughout the interaction
Greenhouse Gases
greenhouse effect: solar radiation passes through the atmosphere and warms the surface of the Earth. The surface radiates some of this energy as longer wavelength infrared radiation which is absorbed by greenhouse molecules which makes the air warmer, causing it to radiate heat. Some of this radiation is re-radiated back to the Earth’s surface and some back to space
the ability of a molecule to absorb infrared radiation depends on the change in dipole moment that occurs as it vibrates
some greenhouse gases are much more effective than others in absorbing IR radiation
global warming potential: amount of infrared radiation that one tonne of a gas would absorb compared to the amount that would be absorbed by one tonne of carbon dioxide
depends on effectiveness and atmospheric lifetime of the gas
Wavenumbers
absorption of certain wavenumbers of IR radiation helps to identify bonds in a molecule
some bonds can be identified by shapes of their signal
ex: O-H bond is broad, C=O is sharp
hydrogen bonding broadens IR absorption so can be detected
ex: O-H in carboxylic acids have broader absorption
molecules with several bonds can vibrate in many different ways and with different frequencies
complex pattern can be used as a ‘fingerprint’ to be matched
comparison of spectrum of a sample with a pure compound can be used as a test of purity
Nuclear Magnetic Resonance Spectroscopy (NMR)
nuclei of atoms with an odd number of nucleons (H, C, F) have a property called nuclear spin and behave like tiny bar magnets
when placed in an external magnetic field, these nuclei can exist in two distinct energy levels
depending on whether magnetic field is aligned with/opposed to the external magnetic field
energy gap between the energy levels is very small and only requires absorption of low-energy radio waves to close the gap between energy levels
as electrons shield nucleus from full effects of external magnetic field, differences in electron distribution produce different energy seperations between the two spin energy levels
nuclei in different chemical environments produce different signals
proton = hydroegn because hydrogen has 1 proton
Magnetic Resonance Imaging (MRI)
application of NMR spectroscopy
uses H’s magnetic moment
with a powerful magnet, radio waves are used to generate an electronic signal that can be decoded to produce images
useful in diagnosis of living tissue due to hydrogen in water
H NMR Spectroscopy
NMR provides:
number of signals in the spectrum
position/chemical shifts of each signal
size/integrated area of each signal
splitting pattern observed for each signal
gives information on chemical environments and therefore structure
Chemical environments
hydrogen nuclei (protons) that have the same chemical environment are said to be equivalent as they give the same signal in NMR
number of signals observed therefore depends on number of chemical environments
Chemical shifts
position where a signal appears in NMR spectrum is measured in terms of chemical shift which has units of parts per million (ppm)
the closer a hydrogen atom is to an electronegative atom, the more pronounced the electron-withdrawing effect and the higher chemical shift observed
the high electronegativity effectively pulls electrons away from the hydrogen atoms thus deshielding the hydrogens’ nuclei
nuclei are now more susceptible to effects due to external magnetic field
hydrogen nuclei in particular environments have characteristic chemical shifts
found in section 21 of data booklet
Splitting patterns
individual signals in NMR do not consist of a single peak
signals are split/resolved into distinctive patterns
splitting occurs as the effective magnetic field experienced by particular nuclei is modified by the magnetic field produced by neighbouring protons
spin-spin coupling
the number and intensity of lines produced are easily predicted
based on the number of neighbouring hydrogens involved in coupling
number of lines: n+1 → n=number of hydrogen atoms on the neighbouring carbons
intensity: pascal’s triangle
pattern will continue for each additional proton on neighbouring carbons
protons bonded to the same atom do not interact as they are equivalent and behave as a group
protons on carbon atoms not adjacent to each other do not generally interact as they are too far apart for their magnetic fields to interact
alcohol protons (OH) typically do nto engage in spin-spin coupling
signals for OH protons are not split and appear as singlets
OH protons are not counted when applying the n+1 rule
 Knowt
Knowt