Lab 4 - Synthesis of Biodiesel
NaOH is a strong base and hygroscopic
Saponification - Hydrolysis of a fat or oil by a base such as NaOH yielding salts of fatty acids (basically the synthesis of soap)
By-product is glycerol (a tri-alcohol that is soluble in water)
Soap molecules have a dual nature in regard to their intermolecular forces; They are both hydrophobic and hydrophilic.
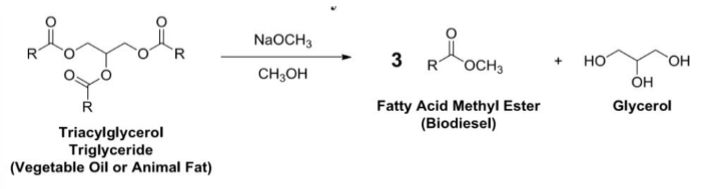
Biodiesel
Is a fatty acid methyl ester formed from transesterification of fats and oils
Fat(or Oil) + Methanol → Glycerol + Fatty Acid Methyl Esters (Biodiesel)
Green Advantages of Biodiesel
Energy source which could replace petroleum
Waste reduction
Byproduct (glycerol) can be used as an alternative feedstock
No net increase in CO2 levels