AP Chem: Chapter 7 - Thermochemistry
Important Vocab
- Thermochemistry: the study of the relationships between chemistry and energy
- Energy: the capacity to do work
- Work: the result of a force acting through a distance
- Thermodynamics: study of energy and its interconversions
- Internal energy: the sum of the kinetic and potential energies of all of the particles that compose the system
- Pressure–volume work: occurs when a force (caused by a change in volume) acts through a distance against an external pressure
- Heat: the transfer of thermal energy
- Thermal equilibrium: Surroundings & object are same temp, no additional net transfer of temp
- Heat capacity: the quantity of heat required to change its temperature by 1 °C
- Calorimetry: measure the thermal energy exchanged between the reaction (defined as the system) and the surroundings by observing the change in temperature of the surroundings
- Enthalpy: the sum of a system’s internal energy and the product of its pressure and volume
Types of Energy
Kinetic
- Associated with the motion of an object
- Ex: Moving ball
Thermal
- Associated with the temperature of an object
- Type of kinetic energy
- Arises from the motions of atoms or molecules within a substance
- Ex: Hot cup of coffee
Potential
- Associated with the position or composition of an object
- Ex: compressed spring, ball held up above the ground
Chemical
- Type of potential energy
- Often stored in chemical bonds
- Associated with the relative positions of electrons and nuclei in atoms and molecules
Thermodynamics
First Law of Thermodynamics
- Also known as the law of energy conservation
- Energy is neither created nor destroyed
- Internal energy: the sum of the kinetic and potential energies of all of the particles that compose the system
- Internal energy is a state system (value depends only on the state of the system)
- Energy flow rules:
- Reactants have a higher internal energy than the products, is negative and energy flows out of the system into the surroundings
- If the reactants have a lower internal energy than the products, is positive and energy flows into the system from the surroundings
Heat
- Heat: the transfer of thermal energy
- Thermal equilibrium: Surroundings & object are same temp, no additional net transfer of temp
- Heat capacity: the quantity of heat required to change its temperature by 1 °C
- Depends on:
- The amount of matter being heated
- Specific heat capacity/molar capacity (q)
- Pressure–volume work: occurs when a force (caused by a change in volume) acts through a distance against an external pressure
- w = F * D
- Calorimetry: measure the thermal energy exchanged between the reaction (defined as the system) and the surroundings by observing the change in temperature of the surroundings
- Measurement tool: bomb calorimeter and coffee-cup calorimeter
- Bomb calorimetry occurs at constant volume and measures ΔE for a reaction
- Coffee-cup calorimetry occurs at constant pressure and measures ΔH for a reaction
Enthalpy
- Enthalpy: the sum of a system’s internal energy and the product of its pressure and volume
- H = E + PV
- Negative delta H = endothermic reaction
- Positive delta H = exothermic reaction
- The value of ΔH for a chemical reaction is the amount of heat absorbed or evolved in the reaction under conditions of constant pressure
- An endothermic reaction has a positive ΔH and absorbs heat from the surroundings. An endothermic reaction feels cold to the touch
- An exothermic reaction has a negative ΔH and gives off heat to the surroundings. An exothermic reaction feels warm to the touch
- Standard heat of formation
- Standard State
- For a Gas: The standard state for a gas is the pure gas at a pressure of exactly 1 atm.
- For a Liquid or Solid: The standard state for a liquid or solid is the pure substance in its most stable form at a pressure of 1 atm and at the temperature of interest (often taken to be 25 °C).
- For a Substance in Solution: The standard state for a substance in solution is a concentration of exactly 1 M.
- Standard Enthalpy Change (ΔH°)
- The change in enthalpy for a process when all reactants and products are in their standard states. The degree sign indicates standard states.
- Standard Enthalpy of Formation ()
- For a Pure Compound: The change in enthalpy when 1 mol of the compound forms from its constituent elements in their standard states.
- For a Pure Element in Its Standard State: delta H = 0
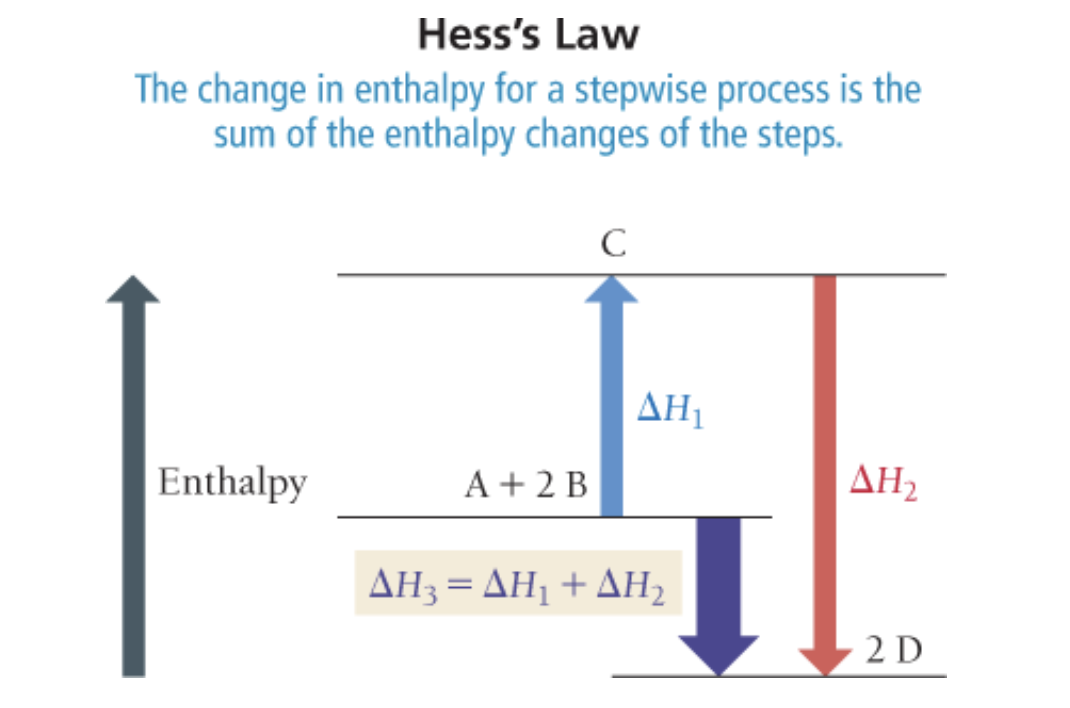
Hess’ Law
- If a chemical equation can be expressed as the sum of a series of steps, then for the overall equation is the sum of the heats of reaction for each step