Cross-Cutting Themes
Topic 1: Microglia – Origin, Functions, and Role in Neurodevelopment
Origin and Turnover of Microglia
Macrophage (e.g., microglia) are characterized by the ability to eliminate foreign cellular material, debris and colloidal material
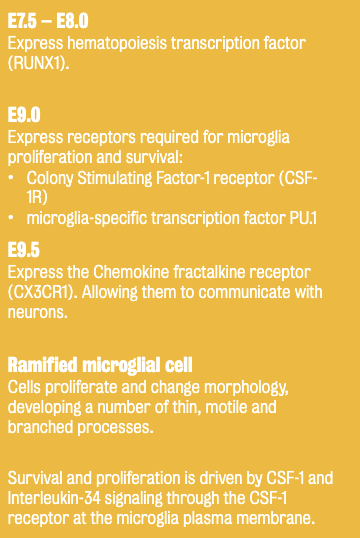
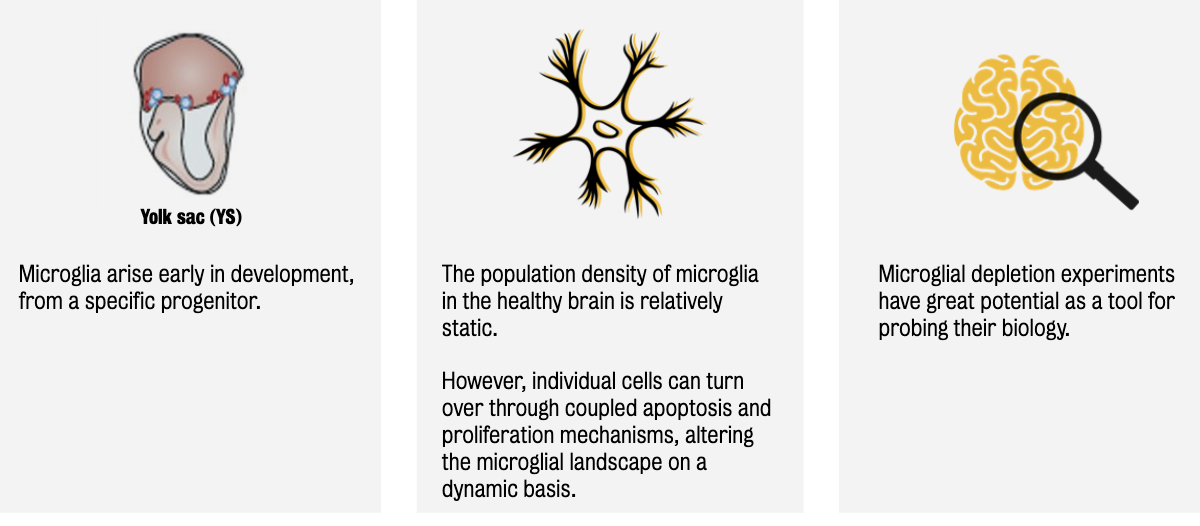
Microglia (tissue-resident macrophages in the CNS) originate from the yolk sac (YS) during early embryogenesis & migrate into the brain.
Represent 5-12% of glial cells in the mouse brain; 0.5-16.6% in humans
Once established, microglia maintain their population through self-renewal rather than infiltration from the periphery.
Experimental depletion (genetic/pharmacological) shows that microglia can be rapidly replaced by local proliferation.
Post-mortem tissues studies in autoimmune disorders reveal that microglial cell numbers increase at foci of disease-related brain insults through the proliferation of brain resident microglia.
If blood-brain barrier is damaged, microglia recruits peripheral monocytes and macrophages
If blood-brain barrier is intact, this state is driven the expansion of brain resident microglia
If the microglia is removed:
Depletion of microglia in the CNS is useful for:

Pharmacological and genetic methods exist to deplete microglia experimentally.
Genetic: use of experimental animals to express an inducible toxin (e.g., diphteria)
Pharmacological: inhibition of the CSF-1 receptor (control microglial survival) using a CSF-1 antagonist (e.g., PLX3397).
Both approaches have side effects, including cytokine release and astrogliosis, but once treatment stops, microglia repopulates the brain within 3-5 days.
Microglial depletion studies suggest that the number of resident microglial cells can be rapidly reconstituted by the proliferation of resident cells, suggesting that microglia are a dynamic population.
Microglial density remains stable over a mouse or human’s lifetime; however, microglia turn over several times during a lifetime (in both mouse and human brain).
Part 2: Functions of Microglia in Development and Adulthood
Microglia are the ‘immune sentinels of the brain’:
They detect and respond to injury, infection, and damage through morphological changes (e.g., becoming amoeboid), up-regulating surface markers, and releasing immune factors (cytokines, ROS, NO).
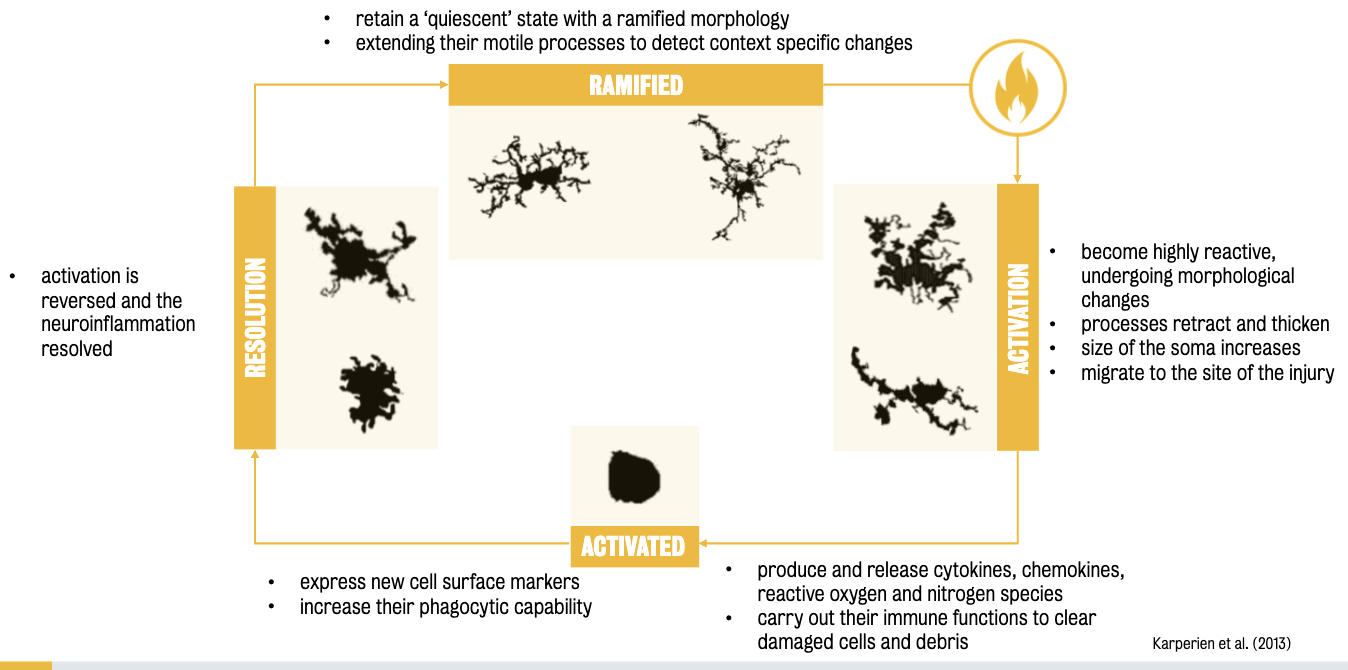
Beyond immunity, microglia serve the following purposes:
Surveillance: Constantly monitor the CNS environment.
Phagocytic microglia: Detect and remove dying cells and debris.
Synaptic Pruning: Eliminate redundant synapses during development—critical for sculpting neural circuits.
Plasticity and Neurogenesis: Involved in hippocampal neurogenesis, experience-dependent plasticity, oligodendrogenesis, and the formation of functional neural networks.
:. Microglia play key roles in sculpting neural circuits during development and maturation of the brain.
Any loss or excess of these roles may contribute to disorders in which neural circuit dysfunction is implicated (e.g., autism and schizophrenia).
In neurodegenerative disorders, microglial activation can be prolonged leading to further neuronal or glial cell death (i.e., astrocytes and oligodendrocytes).
Synaptic pruning during brain development and maturation involves the elimination of some of the synapses forming in early development, while a subset are maintained and strengthened.
Evidence suggests that microglial might play a role in this form of synaptic remodelling and plasticity in the healthy brain:
In vivo 2-photon imaging led to the discovery that microglia have a surveillant role in monitoring the functional state of neurons.
Synaptic engulfment measures in KO mice show that microglia’s role in synaptic pruning is partially mediate through fractalkine signaling (via Cx3cl1, whose receptor is essential for microglial migration).
Relevance: Pathological reductions in microglial activity during neurodevelopment lead to reduced synaptic pruning during adolescence, increasing risk for social or cognitive problems in later life.
Human evidence still lacks; hypothesis remains under investigation.
Part 3: Microglia and Synaptic Pruning in Development
Microglial Dysregulation and Schizophrenia
4 Lines of Evidence:
Post-mortem studies: Mixed findings—some show increased microglial activation; others show no change.
This may be the consequence of:
Use of different microglial markers
Variability in brain region analyzed
Varying methodological approaches
Tissue from patients at different stages of illness
Confounding variables (e.g., age-related lesions, exposure to antipsych. med)
In vivo PET imaging (TSPO binding) suggests activation but not conclusive; TSPO is not specific to microglia.
Represents only current way to supposedly image microglia in the living brain.
Peripheral cytokines studies suggest increased levels of pro-inflammatory cytokines in patients.
GWAS have identified >100 loci implicated in schizophrenia
Antipsychotics and Microglia:
Antipsychotics = mainstay of pharmacological treatment for positive symptoms (e.g., delusions, hallucinations).
PET Study Findings: Antipsychotic-naïve patients show less TSPO binding than medicated ones—suggesting medication itself might increase microglial activity.
Animal Studies: Chronic antipsychotic treatment increases IBA1+ microglia and alters their morphology (larger soma, fewer processes).
Conclusion: Medication is a confounding factor in interpreting microglial activation in schizophrenia.
The Complement System and Excessive Pruning
C4 and CR3 Involvement:
C4 activates complement C3, tagging synapses for removal by microglial CR3.
Genetic variations in C4 may lead to aberrant pruning when combined with stress or infection.
"Two-hit" Hypothesis:
First hit: Perinatal stress/immune activation primes microglia.
Second hit: Later-life stress (e.g., trauma) triggers hyperactive pruning in vulnerable brain regions (PFC, hippocampus).
Results in negative symptoms and cognitive deficits.
Therapeutic Implications
Current Antipsychotics primarily target dopamine D2 receptors—ineffective for cognitive/negative symptoms.
Emerging Treatments:
Immune-targeting drugs (e.g., minocycline, monoclonal antibodies).
Need to balance immune modulation with preserving physiological microglial functions.
Trials underway, but heterogeneity in schizophrenia limits generalization.
Topic 2: Using iPSCs to Model Schizophrenia
Part 1: Clinical Background and Treatment Challenges
Schizophrenia = worldwide public health concern.
Found across all countries and cultures
Lifetime prevalence rate: 0.5.% - 1%
Men have earlier onset (16-25 of age) than women (25-35)
Chronic condition
Current treatment avenues include antipsychotic drugs and behavioural therapies
Symptoms:
Positive: Hallucinations, delusions.
Negative: Anhedonia (lack of ability to experience pleasure), lack of motivation.
Cognitive: Memory, executive dysfunction.
Treatment Limitations:
Antipsychotics only help with positive symptoms.
Side effects include motor issues, metabolic disorders, and immunosuppression.
Many patients (1/4) remain symptomatic, highlighting need for novel approaches.
Part 2: Neuropathology and Genetic Insights
Neuropathological Evidence:
Post-mortem studies found reduced dendritic spine density in schizophrenia brains.
Fewer dendritic spines on cortical neurons suggests disrupted synaptic connectivity and aberrant neural circuit wiring in schizophrenia brains.
Spine Functionality:
Crucial for synaptic transmission and plasticity.
Alterations likely disrupt normal neural information flow, which could result in cognitive deficits seen in brain disorders.
Genetic Risk:
Involves both rare and common variants (e.g., synaptic proteins, dendritic spine regulators), some with weak and others with strong effects
Several genes linked to SZ encode for proteins found at synapses and dendritic spines, exerting powerful regulatory effects on spice structure and function
Mutations in these genes can cause a reduction in the expression of synaptic proteins, potentially affecting the function or structure of dendritic spines
Over 100 common variants identified.
Modelling synaptic deficits in SZ
Approaches:
Animal models — Knockout (KO) mice
Primary neuronal cultures — cells grown in a dish
Using both approaches allow manipulation of gene expression to understand the role of specific protein in synapse structure/function
Part 3: Modeling with iPSCs
While animal models & primary cultures are useful, they don’t fully recapitulate human disease complexity.
iPSCs:
Derived from patient cells; reprogrammed to become neurons.
Can be genetically edited (e.g., to introduce/remove mutations).
Allow direct study of patient-specific synaptic abnormalities.
Isogenic Controls: Created by gene editing to ensure identical background except for mutation of interest.
Brennand et al. (2010) — 1st study to use patient-derived iPSCs to model the SZ.
Cells generated from patient-specific iPSCs display cellular phenotypes directly related to the patient’s genetic background
Electrophysiological recording’s failure to detect any changes in glutamatergic transmission demonstrates that iPSCs cannot visualize all aspects of SZ
Study fails to reveal any insight into the underlying pathophysiology of SZ
Establishes that iPSCs are better suited to model early stages of SZ (prodromal stages).
Yoon et al. (2014):
Used patient-derived iPSCs to visualize SZ-related early neuronal developmental deficits through 15q11.2 CNV.
Findings associate CYFIP1 gene, which regulates cytoskeletal dynamics, with the formation of neural rosettes.
Patient-derived NPCs lines form neural rosettes displaying incomplete PKCgamma rings & reduced adherent junctions, indicating apical-basal polarity & defects in adherens junctions
Conclusion:
15q11.2 alterations play a crucial role in the development of neural rosettes (late-stage progenitor cells generated by iPSCs)
Structural abnormalities observed in patient-derived cell lines reflect early neurodevelopmental changes associated with schizophrenia
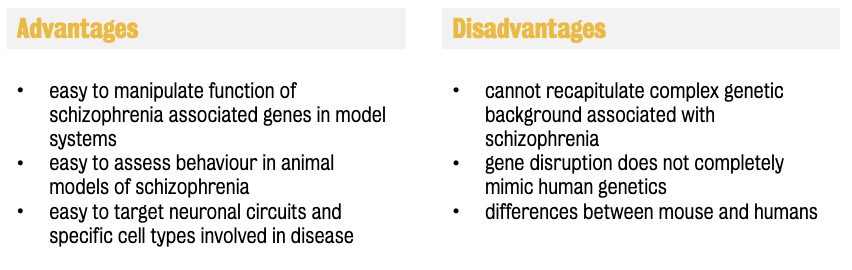
Disadvantages of disease models:
Cannot recapitulate the complex genetic background associated with schizophrenia
Gene disruptions do not completely mimic human genetics
Topic 3: Advances in Stem Cell Technology in Neurodevelopmental Disorders
Part 1: Gene Editing Techniques
These techniques can be used to create isogenic (i.e., they have identical genetic background, safe for the gene modified) iPS cell lines, which can prove a specific gene can cause a disease.
Genome editing can used to:
Edit/modify a genome to recapitulate mutations seen in human patients.
Repair mutations & study resulting phenotypes
Genome editing techniques:
Homologous recombination (early method).
Zinc Finger Nucleases (ZFNs)
Uses artificial enzymes to bind to a specific site on a DNA molecule, then severs it.
Expensive
Not very precise
Sensitive to DNA methylation
Transcription activator-like effector nucleases (TALENs)
Restricition enzymes engineered to cut specific sequences of DNA
Contains non-specific Fok1 nuclease domain
Tedious (each Talen protein needs to be made to target sites)
CRISPR/Cas9
Most efficient and widely used today.
Can be used to investigate disease phenotypes & studying multi-genetic effects
Mechanism: Create double-stranded DNA breaks and repair via:
Non-Homologous End Joining (NHEJ) – knockout genes.
Homology Directed Repair (HDR) – precise edits or corrections.
Benefits:
Test gene function.
Repair patient mutations.
Study effects of multiple mutations (gene-gene interactions).
Part 2: Applications and Limitations
Use Cases:
Recreate patient mutations in healthy iPSCs.
Repair disease mutations in patient iPSCs.
Study resultant neuronal phenotypes.
Challenges:
Time-consuming.
Difficult to model polygenic conditions completely.
Interpretation complicated by variability across lines.
Outlook:
Essential for identifying mechanisms and testing therapies for neurodevelopmental disorders.
Provides unprecedented access to patient-derived human neurons for translational research.

WEEK 5 – KEY TAKEAWAYS
Microglia have critical roles in immune defence and brain development, including synaptic pruning.
Aberrant microglial function and synaptic pruning may contribute to SZ pathophysiology.
Schizophrenia likely arises from complex interactions between genes, environment, and immune activity.
iPSC and gene editing technologies offer promising models to study and treat neurodevelopmental disorders.
New therapeutic approaches targeting microglia and neural circuits are on the horizon but require careful validation.