KAP Chem: Solutions
Lesson 1; Net ionic and other stuff xd
Doble Replacement Reactions / Precipitate Reactions: when in aqueous solutions of dissolved ionic compounds, these reactions can yield insoluble products called precipitates. If no precipitate is form (2 aqueous solutions) there is no reaction.
This reactions can also take place when an acid (has H) and a base (has OH) react to produce a salt (ionic compound that dissolves in water) and water. EX: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Dissociation: When a compound made of ions dissolves in water, the ions separate from one another. EX: NaCl (s) → Na+(aq) + Cl-(aq)
Molecular Equations: each substance is a molecule.
Ionic equation: we separate each compound, leaving each element alone (exept for the precipitate), we add charges and make sure that it is balanced.
Net ionic equation: We erase any ions that were not involved in forming the precipitate or molecule. (Spector ions)
Lesson 2; Water
Lewis Structure of Water: Tetrahedral - Bent molecular shape - polar EN: 1.4
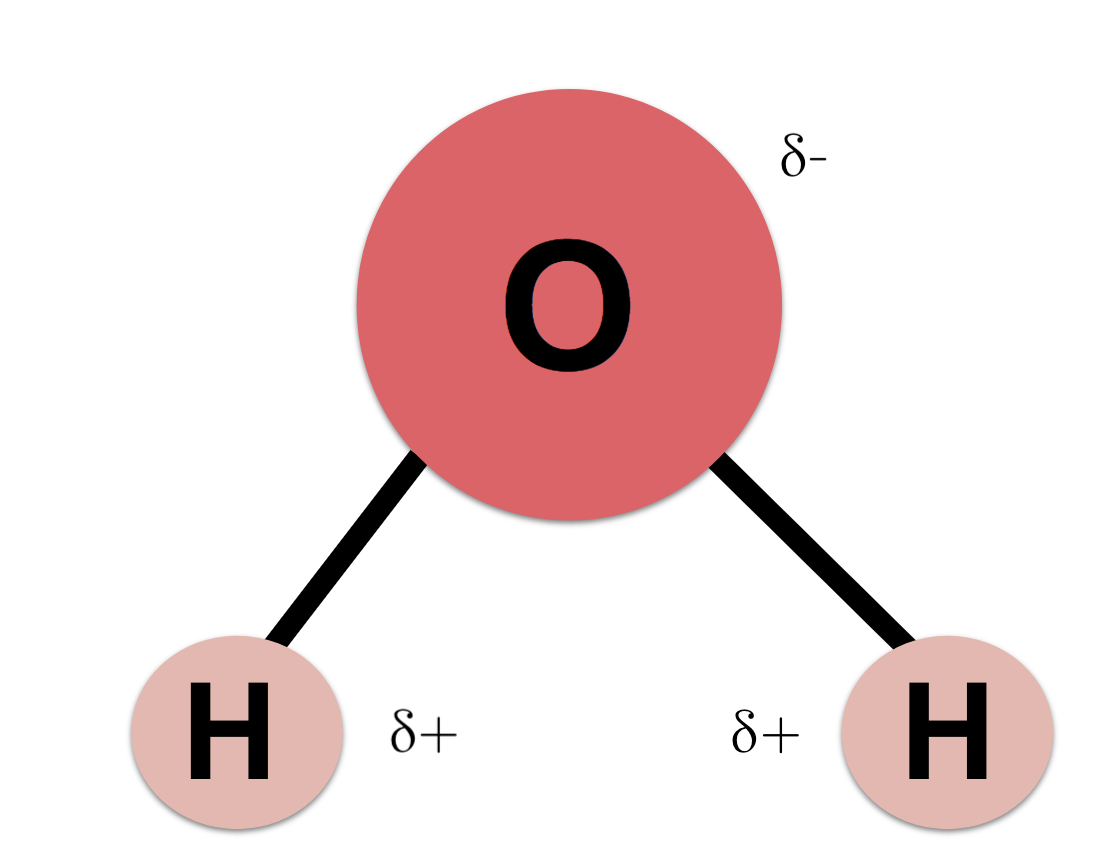
Water has unique physical properties compared to all other compounds.
Hydrogen Bonding
Oxygen is attracted to hydrogen and vice versa, fro strong hydrogen bonds between water molecules. Hydrogen atoms are small which enables them to occupy small spaces, fortifying the IMF. This helps to explain the majority of water unique properties.
Physical Properties
Water has very unique physical l properties when compared to all other compounds.
Liquid at room temperrature
High boiling / Melting / Freezing points: In the case case of boiling the conversion of liquid phase to gaseous phase s the result of pulling apart against intermolecular forces of attraction; the greater the attraction the greater the more energy is needed.
High Heat of Vaporization: refers to the amount ofenergy needed to change 1g of a liquid substance to a gas at constant temperature. The hydrogen bonds have to be broken in order to the molecules to fly off as a gas, which is why it needs high heat of vaporization.
High Specific Heat: refers to the ammoun of energy needed to raise the temperature of 1g of a substance by 1 celsius. You need to break the hydrogen bonds so it requires a lot of energy.
High surface tension: (cohesion: waters molecules affinity for one another through hydrogen bonding/the sticking together of particles of the same sustance)
Density: The solid state of water is lss dense than the liquid state of water.
Solvent: Water is the universal solvent because it easily dissolves polar molecules, ionic compounds and gases more than any other known liquid. Due to this, pure water is not found in nature.
Lesson 3: Solution Features
Solution: homogeneous mixture of two or more substances in a single phase.
When something dissolves it becames uniformly distributed among the water molecules and the solid is no longer visible.
All mixtures have an indifinite composition.
(True) Solutions
Homogeneous mixtures
Cannot be separated by filtration
Not affected by gravity
No Tyndall effect
Particle size more than 1.0 nm
Colloids
Heterogeneous mixtures
Medium size of molecules
Cannot be separated by filtration
Particles remain suspended throughout the solvent from Brownian motion, which is the chaotic movement of the surroundings molecules within the surroundings.
Exhibit the tyndall effect
Can be solid, liquid, or gas mixed with ay other phase.
Suspensions
Heterogeneous Mixtures
Particles are larger compared to the colloids ad solutions
Can be separated by filtration
Affected by gravity, so the settle over time
Exhibit the Tyndall effect (when suspended)
Solvation
Refers to the action of a solute dissolving into a solvent until the solution is stable. (When water is the solvent it is called hydration ad the solution is an aqueous solution.
Electrolyte; substances that dissolve in water to give a solution that conducts electric current.
Any soluble ionic compound
The ions dissociate and are free to move
All soluble salts and ionoc bases are strong electrolytes
Ionization: when covalent compounds dissolve in water to produce ions.
Most covalent compounds do not ionize
Acids are an exception, they produce hydrogen or hydronium ions in the solution.
Strong acids (ionize completely) are strong electrolytes.
Nonelectrolyte; a substance that dissolves in water which does not conduct electric current.
Most covalent compounds except acids.
The neutral solute molecules do not contain mobile charged particles (ions), so they cannot conduct electric current.
Lesson 4: Solubility Behavior
Solubility
Is defines as the amount of the solute that can dissolve in a particular solvent at a given temperature and pressure.
Affected by the nature of the solvent
Polar solves polar
Non polar solves nonpolar
Most ionic compounds will dissolve in water, but do not dissolve in non polar solvents.
Solids: As temperature of a solution increases, so does the solubility. not affected by pressure.
Gases: As temperature increases, solubility decreases. As pressure increases, solubility increases.
Rate of Solubility
How fast a solute dissolves in a given solvent. Factors that affect it:
Temperature; As temperature increases ROS increases
Agitation (stirrring); more contanct with surface of solute, solute dissolves faster.
Solute surfae area: Smaller particles offer greater surface area, so it dissolves faster.
Lesson 5: Solubility
At a given temperature, there is a limit to the ammount of solid that can be dissolved in the liquid solvent. When maximum solubility is reached, molecule are returning to the solid form at the same rate at which they are going into solution. This is called solution equilibrium or dynamic equilibrium; the dissolution and crystallization of a solute occur at the same rates.
Saturated Solution: Contains the maximum ammount of solute for a given amount of solvent at a given temperature.
Unsaturated Solution: contains less solute than a saturated solution at a given temperature.
Supersaturated Solution: a solution that contains more solute than it should be able to hold at a given temperature. is very unstable.
When it is cooled, the excess solute comes out of the solutionor sometimes not.
Lesson 6: Molarity and Dilution
Dilute Solution: Contains a low concentration solute
Concentrated solution; contains a high concentration os solute