Biology Notes (Thanks Anthony and Daniel)
(1st Quarter of Biology)
- Water/Polarization Water / Polarity Quizlet
- Four Macromolecules Macromolecule Quizlet
- Cell Organelles Cell Organelles Quizlet
- Cell Transportation Cell Transports Quizlet
(2nd Quarter of Biology)
- Cellular respiration
- Photosynthesis
- Cell Cycle
- Cancer
(3rd Quarter of Biology)
- Genetic
WATER
Facts
- Water is made out of H2O ( 2 Hydrogens & 1 Oxygen)
- All plants & animals need this to survive.
- Water is a polar molecule which is when one side is negative and the other side is positive.
- Its polarity makes water a great solvent or a universal solvent. It is called this because it has the power to dissolve many substances other liquids can't do.
- Oxygen is a partial negative charge and hydrogen is a partial positive charge. Water is unevenly charged.
- When water is not attracted to something it does not move and when it is attracted to something it can move.
Vocab
Solution - When the substance in the water is fully dissolved and leaves no trace.
Cohesion - Water is attracted to water.
Adhesion - when water is attracted to other substances.
Polar Covalent Bonds
These are chemical bonds that are when the electrons are shared unequally between the molecules.
Nonpolar Covalent Bonds
These are chemical bonds that are when the electrons are shared equally between the molecules
Hydrogen Bonds
Hydrogen bonds are when water bonds to each other like a pool. The water molecules’ oxygen will connect to hydrogen and then create a bond. This can happen in a big area and create a small film at the top. If your density is lower than the film you can stand on it that is why some bugs can stand on water.
THE FOUR MACROMOLECULES
Carbohydrates - Glucose
Lipids - Glycerol, Fatty Acids,
Proteins - Amino acids, Glycine \n Nucleic Acids - Cytosine
The structure of the molecules determines their function.
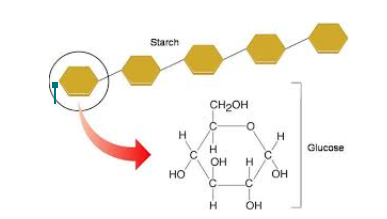
CARBOHYDRATES
Facts
- Made up of carbon, hydrogen, and oxygen (CHO)
- Includes simple sugars like glucose, fructose, and galactose
- It also includes more complex molecules like starches and cellulose(plant fibers).
- Broken down by the body into glucose. Glucose is used as fuel by your body's cells, tissues, and organs.
- Each time a glucose
- The ratio of 1:2:1
Function
- Living things use carbs as their main source of energy. Plants, some animals, and other organisms use carbs for structural purposes.
Simple Sugars
- Single sugars like glucose are monosaccharides
- Fructose is a simple sugar with the same chemical formula as glucose
- -sucrose is a disaccharide, a compound made by joining 2 simple sugars together (using dehydration synthesis)
Complex Carbohydrates
- Polysaccharides are large macromolecules formed from monosaccharides
- Animals store excess sugar in a polysaccharide called glycogen, known as animal starch
- When blood sugar runs low, glycogen is broken down into glucose and then released into the blood
- plants have a different polysaccharide known as starch, to store excess sugar.
- Plants also make another important polysaccharide called cellulose
- The thought & flexible cellulose fibers give plants much of their strength and rigidity.
Relationships
- Cows have a symbiotic relationship with enzymes.
- Cells burn glucose and store the energy as ATP.
Formulas
Glucose + Fructose = Sucrose + H2O
Glucose + Glucose = Maltose + H2O
Glucose + Galactose = Lactose + H2O
LIPIDS
Lipids are composed of a long hydrocarbon chain.
Diverse Group
- Fats
- Phospholipids
- Steroids.
Fats
- Glycerol + 3 fatty acids
- Long HC chain
- Non-Polar
- Hydrophobic
Functions
- Energy storage
- Very rich
- 2x energy in carbos
- Cushions organs
- Insulates body
Fatty Acids
- Be the same or different in one molecule
- Vary in length
- Vary in number/location of double bonds
- Saturated are Single bonds
- Unsaturated is double-bonded.
- Fats usually form when glycerol molecules combine with fatty acids
- Lipids have an oily fatty acid that interacts strongly with water
- 3 elements make up fatty acids: carbon, oxygen, and hydrogen
Lipid Bilayer
- Gives cell membranes a flexible structure that forms a strong barrier between the cell and its surroundings
- The cell membrane regulates what enters and leaves the cell and also protects and supports the cell
Saturated Fats
All carbon bonded to hydrogen
No C=C double bonds
- Long, Straight chains
- Most animals fats
- Solid at room temp
Unsaturated Fats
C=C double bonds in the fatty acids
- Plants and fish fats
- Vegetable oils
- Liquid at room temp
If more double bonds are present it reduces the number of hydrogen atoms in the fatty acids.
Hydrophobic Vs. Hydrophilic
- The fatty acid part of the lipid is hydrophobic (tails)
- The opposite end of the molecule is hydrophilic (heads)
- Hydrophilic is “water-loving” while hydrophobic is “water-hating”
- Tails cluster together while the head attracts the water
- Fatty acid tails form an oily layer inside the membrane (keeps water out)
Glycerol
- Considered as alcohol
- Glycerol + fatty acid = lipids
- One of the two molecules that make up a monomer known as triglycerides
Phospholipids - 2 fatty acids instead of 3
PROTEINS
Facts
- Most diverse out of all the biological molecules
- Due to the Amino acid, it can be so diverse.
- Polymers of molecules called amino acids.
Proteins
- Amino acids are compounds with an amino group (-NH2) on one end and a carboxyl group (-COOH) on the other end.
- Peptide bonds link amino acids - create polypeptides.
- Some proteins control the rate of reactions and regulate cell processes. Others form important cellular structures, while still others transport substances into or out of cells or help to fight disease.
Structure & Function of Proteins
- Storage: proteins that serve as reserves of amino acids and metal ions. An example would be egg whites. It can be maintained as growth for organisms.
- Transport: proteins that are found in your membrane and move your body materials.
- Regulatory: proteins that protect your body's DNA sequence and increase or decrease the production of hormones.
- Movement: proteins that allow your muscles to work.
- Structural: proteins that are responsible for your cell shape and provide support for many things like bones, hair, and nails.
- Enzymes: proteins that make cellular reactions. They can help speed up chemical reactions and metabolism in our bodies. They can also make substances and break others down as well.
Four Levels of protein structure
- Primary structure: Amino acids bonded together by peptide bonds (straight chains)
- Secondary structure: 3- dimensional folding arrangement of a primary structure into coils and pleats heal together by hydrogen bonds: coils and pleats
- Tertiary Structure: Secondary structure bent and folded into a more complex 3-D arrangement of linked polypeptides. It folds due to H-bonds, ionic, and disulfide bridges (S-S) It is also called a subunit.
- Quaternary Structure: Composed of 2 or more subunits, Globular in shape, formed in Aqueous environments. An example is enzymes(hemoglobin)
Levels of Organization
- Amino acids are assembled into polypeptide chains according to the instructions inside the DNA.
- Proteins have four levels of organization/structure.
- A protein's primary structure is the sequence of its amino acids.
- The secondary structure is the folding or coiling of the polypeptide chain.
- The third/tertiary structure is a polypeptide chain's complete, three-dimensional arrangement.
- Proteins with more than one chain are said to have a fourth level of structure, describing the way in which the different polypeptides are arranged with respect to each other.
- The shape of a protein is maintained by a variety of forces, including ionic and covalent bonds as well as the van der Waals forces and hydrogen bonds.
- Protein structures are VERY IMPORTANT.
- Proteins HAVE to be in the TERTIARY STRUCTURE to be functional.
Proteins are able to be Denatured by something that is highly acidic. This can make the protein lose its structure therefore its function.
NUCLEIC ACIDS
What are Nucleic Acids?
- Large macromolecules found in all cells and viruses
- Main types: DNA & RNA
- Made up of carbon, hydrogen, oxygen, nitrogen, and phosphorus (CHONP)
- Monomers of nucleic acids: nucleotides
- Individual nucleotides join by covalent bonds to form a nucleic acid
Function of Nucleic Acids
- Dictate the sequence of amino acids
- Gives genetic information to chromosomes, which is then passed from parent to offspring, as seen in the biological dogma, DNA → RNA → Protein
- Store & transmit heredity, or genetic, information
- Two kinds of nucleic acids: DNA & RNA (the name RNA consists of sugar ribose and DNA consists of sugar deoxyribose)
DNA (Deoxyribonucleic Acids)
- Made up of deoxyribose sugar, phosphate groups, and one of the 4 bases
- 4 different types of bases: Cytosine, Adenine, Guanine, and Thymine
- Structure: has a double-stranded helix structure with two sugar-phosphate strands connected by hydrogen bonds in the nitrogenous bases
RNA (Ribonucleic Acids)
- 4 different types of bases: Cytosine, Adenine, Guanine, and Uracil
- Uracil is found in only RNA while Thymine is found only in DNA.
- Structure: a single-stranded helical figure.
Structure
- 3 parts to nucleotides: A 5-carbon sugar (pentose), a Nitrogen-containing base made up of Carbon, Hydrogen, & Nitrogen, and a phosphate group.
- The structural model of RNA is composed of 1 sugar-phosphate strand, and is single-stranded, making the sugar-phosphate backbone the entirety of its structure.
- The structural model of DNA is composed of 2 sugar-phosphate strands connected together by hydrogen bonds between the nucleotide bases, forming a double helix.
BACTERIA
Bacteria
- Bacteria are mostly good but some are bad
- Bacteria compete with fungus.
Bacterial Quorum Sensing
CELL ORGANELLES
- NUCLEUS
Function
- Contains eukaryotic cell’s genetic library
- Most genes in the nucleus
- Some genes are located in mitochondria and chloroplasts
Size
- 5 Micron (µm) in diameter
Facts
- The copy of the DNA is a messenger RNA (mRNA) which goes into the ribosomes which is where proteins are made.
- Nuclear Envelope
- Double Membrane is fused in spots farming Nuclear Pores
- Nuclear Lamina - netlike array of protein filaments on the nuclear side of the envelope that maintains the shape of the nucleus (plays a role in reforming nuclear membrane after cell division, if you inject antibodies to lamina proteins, nucleus can’t reform after mitosis.)
B. CELL MEMBRANE
Function
- Separates the interior of the cell from the outside environment.
- Regulates the transport of materials entering and exiting the cell.
- Regulates homeostasis.
Facts
- The terms “cell membrane” and “phospholipid bilayer” are interchangeable.
- Phospholipid bilayer processes all cell transportation into and out of the cell.
- Uses protein channels to help some molecules to pass through including water, and anything polar.
C. RIBOSOMES
Facts
- Prokaryotic Cells & Eukaryotic Cells have different ribosomes
- Different size subunits
- Different proteins
- Ribosomes that make proteins in the ROUGH ER are made to LEAVE the cell. Ribosomes in the CYTOPLASM make proteins IN/FOR the cell.
D. MITOCHONDRIA
Function
- generates ATP for the cell
- The only reason for cell life
- Programmed Cell Death
- Stem Cell Regulation
- Regulation of Innate Immunity to pathogens.
How It Works
- it uses oxygen available within the cell and converts chemical energy from food in the cell to energy
E. ENDOMEMBRANE SYSTEM
Facts
- Regulates protein traffics and performs metabolic functions in the cell.
Includes
- Plasma membrane
- Nuclear membrane
- Endoplasmic reticulum
- Golgi apparatus
- Vacuoles
- Lysosomes
- Rough ER
F. CYTOPLASM
Function
- The medium for chemical reaction.
- Provide a platform upon which other organelles can operate within the cell.
- The reason why organelles stay inside the cell.
Description
- Translucent gel-like fluid inside the cell.
G. GOLGI APPARATUS
Function
- Help proteins received by the ER to be further processed and sorted for transportation to their eventual destinations.
- Turn lipids into vesicles for delivery to the targeted destination.
H. LYSOSOME (AOC)
Function
- Breaks down and digests proteins, carbohydrates, lipids, and nucleic acids.
- Removal of waste products / dead cells.
- Responds to foreign substances (i.e., bacteria, viruses, antigens…).
- Repairs the cell membrane.
I. CELL WALL (POC)
Function
- Provides tensile strength.
- Protection against mechanical and osmotic stress.
- Give shape to the plant.
- Separates the interior of the cell from the outer environment.
J. CHLOROPLAST (POC)
Function
- Produces the energy for plant cells from photosynthesis.
- Oxygen-release processes.
- Help sustain animal growth and crop yield.
K. VACUOLE
Function
- Sequester waste products (Animals)
- Maintain water balance. (Plants)
CELL TRANSPORT / CELL MEMBRANE
Facts
- Goes from high concentration of molecules to a low concentration of molecules.
- Protein channels of the bilayer help some molecules to move through the membrane which is polar and any fully charged molecules.
- Moving with the concentration gradient means that the diffusion of the cells is going from high concentration to low concentration.
Vocabulary
- Concentration Gradient - The gradual change in concentration over distance in solutions over time.
- Simple Diffusion - Does not require the Transport of Protein tunnels.
- Facilitated Diffusion - Requires the transport of proteins.
- Endocytosis - The process by which molecules gain something.
- Exocytosis - The reverse of exocytosis (molecules lose something).
- Active Transport - When cells move through the cell membrane AGAINST the concentration gradient, requires ATP/energy, to pass through the cell membrane, examples include running up a hill.
- Passive Transport - When cells move through the cell membrane WITH the concentration gradient, requires no energy, examples include a ball or car rolling down a hill.
CELL THEORY
The 3 Theorems
- All living things are made up of at least one or more cells.
- A cell is the basic unit of structure for all living organisms.
- All cells must come from other already pre-existing cells.
Q1 EXAM REVIEW
Vocabulary
- Quantitative Controlled Experiment - A controlled experiment in which numbers are involved for the information. Something that is quantity based.
- Independent Variable - Something that doesn’t need anything in order to operate.
- Dependent Variable - Something that must first need another thing in order to operate. (depends on something)
- Characteristics of Life - the ability to reproduce, homeostasis, growth and development, energy use, ability to adapt, respond to the environment, and cellular organization.
- Evolution - A change in which children of animals/ plants gained new characteristics to help adapt to the new changes in life.
- Cell Homeostasis - Cell homeostasis is the process that help maintain the cell at an internal steady state.
- Sexual Reproduction - When two organisms of the same race reproduce by sexual organs.
- Response to Stimuli - When an organism responds to a change of nature. Something like movement, sweat, and something that happens due to your body changing.
- pH Scale - Scale that measures the amount of acidity in an object. Anything below 7 is an acid and anything above 7 is a base. 7 itself is water.
- Enzymes - Catalysts to chemical reactions within the human body.
- Environmental Factors for Enzymes - Temperature, pH, and concentration can all affect the function of enzymes in many ways.
- Lock and Key Model - When an enzyme requires a certain substrate in order for it to operate.
- Enzyme/Substrate Complex - When a substrate binds to an enzyme, causing the enzyme to clamp down on the end of the substrate in order for it to fit.
- Hypotonic - Low concentration of matter.
- Osmosis - When an object such as water is trying to exit the cell membrane through a high concentration gradient to a low concentration gradient.
- Hypertonic - High concentration of matter
- Isotonic - When the matter is equal on both sides.
- What process is always used when creating and completing an experiment?
Scientific Method
- Describe the difference between qualitative and quantitative data. Give 2 examples for each.
Qualitative data are general observations. Ex: Color changed from yellow to green. Quantitative data is with numbers. Ex: length of stem, length of paper, volume of something
- How many variables can be changed during an experiment?
One
- What is the name for the variable that gets changed?
Independent variable
- What is the name for the responding or measured variable?
Dependant variable
- What is the correct format for a hypothesis?
If, then, and because statement
- Create a hypothesis about how chocolate gives you pimples.
IF you eat chocolate THEN it will give you pimples BECAUSE it is unhealthy
- What are the common characteristics of ALL living things?
Genetics, Cells, Evolution, Responds to Stimuli, Homeostasis, Grow and adapt, reproduction, use energy
- What is the vocab word for a stable internal environment?
Homeostasis
- What are the four macromolecules? - Proteins, Carbohydrates, Nucleic Acids, Lipids
- What are the monomers for the macromolecules? - Proteins=Amino Acids, Nucleic Acid- Nucleotides, Lipids- fatty acids, Carbohydrates=Monosaccharide.
- Enzymes are examples of which macromolecule? - Proteins
- What is the function of carbohydrates? - To provide short term energy for the body.
- What are the two types of nucleic acids? - DNA & RNA
- What are the differences between Eukaryotes and Prokaryotes? - Eukaryotes: DNA in nucleus, have membrane bound organelles. Prokaryotes: DNA in cytoplasm, do not have membrane bound organelles.
- What are 5 differences between plant and animal cells? - Plant cells: cell wall, chloroplasts, large water vacuoles. Animal cells: have centrioles
- What is another name for the cell membrane? - Phospholipid Bilayer
- What molecules can freely pass through the cell membrane? - Water and oxygen
- Which type of transport does NOT require energy? - Passive Transport
- In which direction do molecules move in simple and facilitated diffusion? - Higher to Lower concentration
- What large molecules can be embedded in the cell membrane and help move molecules? - carrier proteins, channel proteins, ion channels, aquaporins
- What is the diffusion of water called? - Osmosis
- What type of solution is seen when a cell expands or fills with water? - hypotonic solution
- What happens to a cucumber’s cells when placed in a salt/brine solution? What type of solution is that? - It will shrink and look shriveled up, hypertonic
- What is the cellular energy needed for active transport? - ATP
- In which direction do molecules move in active transport? - from low to high concentration
- What elements do all macromolecules have? - carbon, oxygen, hydrogen
CELLULAR RESPIRATION
Facts
- Cellular Respiration produces water, CO2, & ATP.
- Cells expel the CO2 into your blood which is then released through our lungs & breath.
- Cellular respiration happens in all cells, in all living organisms.
- Aerobic Respiration is synonymous with Cellular Respiration.
- Difference between breathing and cellular respiration. They are two extremely
different things.
- Bacteria have the ability to make ATP under anaerobic conditions (under no
oxygen).
- 36 ATP molecules can be made from one glucose molecule.
- 2 ATP molecules are created from fermentation.
- After losing its third molecule, ATP becomes ADP, Adenosine diphosphate.
- Bacteria, Arachae, Yeast, Muscle Cells (To extent) all perform anaerobic
respiration.
- Sulfate is used instead of oxygen in the process of Fermentation.
- LEO GER- Lose electrons oxidized, Gain electrons reduced.
- Acetaldehyde is the derivative of pyruvate and can accept electrons with the
same easiness pyruvate can.
- Photosynthesis makes glucose while cellular respiration breaks it.
- Cellular Respiration can also occur by harvesting chemical energy.
- All the reactions in your body are metabolism.
- Electrons move in steps from carrier to carrier downhill to oxygen, which allows for more control for oxidation and release of energy.
Vocabulary
- Respiratory System - The network of organs and tissues that help you breathe. Vital organs within this system would include nose, throat, larynx, trachea, bronchi, and lungs.
- Glucose - A simple sugar that is the component of many carbohydrates and is vital for the creation of ATP, energy.
- Anaerobic Respiration - The only way that things like yeast can produce energy.
- ATP (Adenosine Triphosphate) - The source of energy for use and storage at a cellular level. The goal of Cellular Respiration.
- Carbon Dioxide (CO2) - A chemical compound formed by molecules that each have 1 carbon atom and double bonded with 2 oxygen atoms.
- Expel - To force out or remove from an object.
- Fermentation - When cells process glucose without respiration. Seen as the backup plan of breathing. Deprived of oxygen.
- Glycolysis - The process in where glucose is broken down to produce energy it does not require oxygen
- Lactic Acid Fermentation - Lactic acid fermentation is a process in which glucose or other six carbon sugars are converted into cellular energy with lactic acid in the solution. It occurs when there is no oxygen.
- Alcoholic Fermentation - A process in which yeasts convert sugars to energy but create ethanol or an alcohol as it is a waste product causing the name Alcoholic fermentation.
- Mitochondria - The cell organelle that processes cellular respiration and produces energy for the body. Called the powerhouse of the cell because it produces all the power for the cell to work.
- Oxidative Phosphorylation - The synthesis of ATP by the adding of one adenosine phosphate to ADP (Adenosine Diphosphate), which is attached by ATP synthase.
- Chemiosmosis - The diffusion of ions across a cell membrane.
- Respiration - To make ATP as well as some heat by burning fuels in many steps.
- Combustion - To make a lot of heat energy by burning fuels in one step.
- Catabolism - Destruction or breaking of something larger into something smaller.
- Anabolism - The building of something smaller into something larger.
- Exergonic - A process that releases free energy.
- Endergonic - A process that absorbs energy.
Chemical Equations
C6H12O6+6O2 ---------> 6CO2+6H2O+ATP= cellular respiration
6CO2+6H2O ------------> C6H12O6+6O2= photosynthesis
LIGHT
THREE STEPS OF ATP
STEP 1. Glycolysis (a remnant of old bacteria) - takes place in cytoplasm, does not
require oxygen. 2 net ATP molecules and 2 NADH molecules made from
conversion. 1 ATP molecule required to convert to pyruvate. Can send net ATP
molecules and NADH molecules to mitochondria.
**STEP 2.**Krebs cycle( takes place in mitochondria): Pyruvate→
acetyl-CoA--->oxidized----> 2 FADH² molecules & 6 NADH molecules.
↓
2 NADH pre krebs cycle
STEP 3. Electron transport chain (takes place in mitochondria)- NADH molecules and
FADH2 push out hydrogen ions to produce a gradient on one side of the
membrane. NADH and FADH2 fall down after pushing out ions due to oxygen
pulling it. Hydrogen bonds with the oxygen pulling it down, creating water.
Harvesting Chemical Energy
Stored Energy
- Stored energy is found within the organic molecules in your body. (Carbs, Proteins, Fats)
- When harvesting stored energy, heterotrophs (animals and all alike), have to eat food in order to gain organic molecules for the body to pick glucose off of.
- Heterotrophs digest these organic molecules so that they can gain materials to make ATP, as well as fuel for energy.
- These fuels are burned in a step-by-step enzymatic reaction.
- The goal of harvesting chemical energy is the catabolism of glucose, the process of breaking up more and more glucose molecules to make ATP.
Energy by Fuel
- Catabolic reaction.
- Digesting larger molecules into smaller molecules.
- This breaks bonds between atoms and moves electrons from one molecule to another molecule.
- As these electrons move between the molecules, they are carrying energy with them.
- This energy is stored in another bond, which is then released as heat or harvested to make ATP.
Redox Reactions & Moving Electrons
- Electrons cannot move alone in cells.
- Electrons in cellular respiration can only be moved when attached to Hydrogen.
- If a Hydrogen atom is moved, then an electron is moved with it.
- In respiration, electron carriers move the electrons by shuttling H atoms around.
- When NAD+ and FAD+2 turn into NADH and FADH2, it means they have been reduced.
- Redox reactions in cellular respiration fuel everything.
- Redox reactions release energy as organic molecules are broken down.
- Redox reactions break the bonds between Carbon - Carbon molecules.
- Redox reactions strip the electrons from Carbon - Hydrogen bonds by removing the Hydrogen atom.
- When glucose turns into carbon dioxide, (C6H12O6 → CO2), it means that the fuel has been oxidized.
- As electrons are attracted to more electronegative atoms, the electrons in the hydrogen atoms taken from the glucose molecule cause the hydrogen atoms to be drawn to oxygen, (the most electronegative atom in biological molecules).
- This, then creates water molecules. When oxygen atoms turn into water molecules, it means that oxygen has been reduced.
Oxidation & Reduction
- Oxidation is a loss of electrons.
- A removal of the Hydrogen ion.
- An oxygen atom takes its place.
- Oxidation releases energy.
- This process is exergonic.
- Reduction is a gain of electrons.
- It is the adding of a hydrogen atom.
- This process removes oxygen from the equation.
- This process ends up storing energy.
- This process is endergonic.
STAGES OF CELLULAR RESPIRATION
STAGE 1: GLYCOLYSIS
Facts
- Glycolysis occurs in the cytoplasm/cytosol of the cell. It does not require oxygen to progress.
- There are 10 reactions within glycolysis.
- Glycolysis produces two molecules of ATP energy, as well as 2 NADH electron carriers.
- Glycolysis consumes/burns two molecules of ATP.
- Glycolysis has a net production of two molecules of ATP, and two electron carriers, NADH.
How It Works
- Glycolysis converts one molecule of glucose, with six atoms of carbon, to two molecules of pyruvate, with three atoms of carbon.
- Glucose is first converted into a molecule called fructose-1, 6bP, or fructose-1, 6 Bisphosphate. Fructose-1, 6bP is a substrate to the enzyme PFK,
- The enzyme PFK converts the molecule into 2 molecules that have 3 atoms of carbon and one phosphate. These molecules can either be called DHAP or G3P.
- The phosphate is then taken away from the molecule and added to ADP to create ATP, thus giving the 2 net ATP.
- With the removal of the phosphate from DHAP/G3P, the molecule is converted into a pyruvate molecule.
STAGE 2: PYRUVATE OXIDATION
Facts
- After glucose is turned into two pyruvates, the pyruvate molecules go into the mitochondria and enter the mitochondrial matrix.
- The oxidation process progresses in three steps.
- The process releases 2 molecules of carbon dioxide (CO2).
- The process reduces 2 NAD molecules into 2 NADH molecules.
- The process produces 2 acetyl CoA, as well as 2 compounds sugars, NADH, and carbon dioxide as byproducts.
STAGE 3: KREBS / CITRIC ACID CYCLE
Facts
- The Krebs Cycle is found in the mitochondrial matrix.
- It follows an 8-step pathway, each step catalyzed by a specific enzyme.
- The Krebs Cycle can be described as a stepwise process of catabolism of 6C citrate molecules.
- The net products of the Krebs cycle are 2 ATP molecules, 8 NADH, and 2 FADH2 molecules.
Importance
- The Krebs cycle produces large quantities of electron carriers such as NADH and FADH2 which go to the Electron Transport Chain.
STAGE 4: ELECTRON TRANSPORT CHAIN
Facts
- The phosphorylation of ADP within ATP Synthase creates ATP.
- Once the hydrogen ions are on the high concentration side of a membrane, they fall back through the protein tunnel (ATP Synthase) and become ATP.
- Electron carriers pass electrons & H+ to ETC
- H cleaves off NADH & FADH2
- Electrons are stripped from H atoms → H+ (protons)
- Electrons passed from one electron carrier to the next in the mitochondrial membrane. (ETC)
- Flowing electrons = energy to do work.
- Transport proteins in membrane pump H+ (protons) across the inner membrane to intermembrane space.
- The electrons from glycolysis and the krebs cycle are doing all the work to move the hydrogen ions into ATP.
- The negative charge from the oxygen molecule pulls the electrons in the membrane down to form a water molecule.
- Where ATP is made by falling through ATP synthase is called oxidative phosphorylation.
PHOTOSYNTHESIS
Photosynthesis
- Light energy is described as photons)
- Anabolic (small molecules combined)
- Endergonic (stores energy)
- Carbon dioxide (Co2) requires a process that uses light energy (photons) and water (H20) to produce organic macromolecules. (glucose)
- Glucose is recognized as an organic compound because of the element Carbon within its chemical formula.
Plants
- Autotrophs - produce their own food (glucose)
- Process called photosynthesis
- Mainly occurs in the leaves:
- Stoma - Pores
- Mesophyll cells
Stomata (Stoma)
- Pores in a plant’s cuticle through which water vapor and gases (CO2 & O2) are exchanged between the plant and atmosphere.
- Carbon Dioxide and other gases pass through the guard cells of the Stoma.
- Stomata is found on the underside of the leaves
Mesophyll Cell of Leaf
- Photosynthesis occurs in these cells.
- Includes: Cell Wall, Nucleus, Central Vacuole, Chloroplast.
- Chloroplasts are filled with a pigment called chlorophyll.
Chloroplast
- Organelle where photosynthesis takes place.
- Thylakoid stacks are connected together within the chloroplast.
- Includes: Inner Membrane, Outer Membrane, Stoma, Thylakoid, Granum.
- Has a double membrane like the mitochondria.
Concentration gradient is made up of hydrogen ions in the chloroplast membrane.
The Hydrogen ions are going to fall through ATP synthase to produce ATP.
THYLAKOID
- Includes: Thylakoid Membrane, Thylakoid Space. Stacked inside Granum. Grana makes up the inner membrane.
CHLOROPHYLL MOLECULES
- Chlorophyll molecules are located in the thylakoid membranes.
- Chlorophyll have Mg+ (MAGNESIUM) in the center
- Chlorophyll pigments harvest energy (photons) by absorbing certain wavelengths (blue-420 nm and red 660nm are most important)
- Plants are green because the green wavelengths are reflected and not absorbed.
- Chlorophyll has an alcohol attached to it.
Wavelengths of Light (nm)
Short wave has more energy than a long wave, which has less energy.
Absorption of Light by Chlorophyll
- Chlorophyll absorbs blue-violet and red light the best.
- With green light, chlorophyll does not absorb, which is why it is reflected back.
Falls Colors
- In addition to the chlorophyll pigments, there are other pigments present.
- During the fall, the green chlorophyll pigments are greatly reduced revealing the other pigments.
- Carotenoids are pigments that are either red, orange, or yellow.
Redox Reaction
- The transfer of one or more electrons from one reactant to another
- There are two types of redox reactions.
- Oxidation is the loss of electrons.
- Reduction is the gain of electrons.
Oxidation Reaction
- The loss of electrons from a substance of the gain of oxygen.
- In the chemical formula of photosynthesis, the oxidation process is found in the reactants of CO2, and 6 oxygen molecules.
Reduction Reaction
- The gain of electrons to a substance or the loss of oxygen.
- In the photosynthesis chemical equation, reduction can be seen in …
PHOTOSYNTHESIS STEPS
Light reaction:
Light and water enter the Thylakoid Stroma.
Light splits the water molecules into hydrogen ions and oxygen. The oxygen created by the light reaction is a byproduct of photosynthesis and is forced out of the cell.
The oxygen from the CO2 that enters the calvin cycle, then makes G3P or glucose.
Calvin cycle:
The calvin cycle has three phases.
2 spins of the Calvin Cycle create ATP.
FIXATION, REDUCTION, REGENERATION
CELL CYCLE
Facts
- 90% of the cell cycle is spent in the interphase. The other 10% is spent during mitosis.
- CELL PLATES ARE ONLY FOUND IN PLANT CELLS.
- In animal cells, the cell pinches in and creates a cleavage furrow
Definition
- Tumor - Hard lump of thousands of cancer cells condensed together.
- Phagocytes - White blood cells
s
Interphase
Dna replication & cell growth
Prophase
Chromosomes become visible, the nucleolus disappears, the nuclear membrane breaks down, and spindle fibers form in the cytoplasm.
Metaphase
Chromosomes line up in the middle, the nucleus disassembles, the spindle fibers pull and push the chromosomes to create a straight line.
Anaphase
Chromosomes move away, towards the poles of the cells by spindles.
Telophase
Chromosomes are on opposite ends of the cell, this causes two new nuclei to form. The spindle fibers disintegrate due to the presence of it not needed.
Cytokinesis
When the cytoplasm splits and create two genetically identical daughter cells.
CANCER
Q2 EXAM REVIEW
Study cellular respiration- phases, what goes in and out, Atp generation,purpose
Photosynthesis- phases, what goes in and out,Purpose
Cancer
Stages of cell cycle
Mitosis-how long it is, can know what phase it on with said image
- What goes into cellular respiration?
Glucose and oxygen
- What are the products?
ATP, Carbon dioxide, water
- What is being recycled in cellular respiration and photosynthesis?
ATP and ADP
- Where does the Carbon dioxide in the mitochondria come from?
It comes from the Krebs cycle
- Stages of Cellular Respiration
- Reactants & Products
- Stages of Photosynthesis
- What & When molecules go into certain parts of Photosynthesis & Cellular Respiration
- Phases of Cell Cycle
- G1, S, G2
- Fermentation
- Mitosis
- Stages of Mitosis
- Cancer
- How long a cell spends in each phase
- Diagram of Mitosis
MENDELIAN GENETICS
Gregor Johann Mendel (1822 - 1884)
- Austrian monk(ey) of Christianity
- Studied the inheritance of traits in pea plants.
- Developed the laws of inheritance.
- Mendel’s work was not recognized until the turn of the 20th century.
- Between 1856 - 1863, Mendel cultivated and tested some 28,000 pea plants.
- Found that the plant's offspring retained traits of the parents.
- Called the “Father of Genetics”
Particulate Inheritance
- Mendel stated that physical traits are inherited as “particles”
- Did not know that the “particles” were actually Chromosomes & DNA.
Genetic Terminology
Trait - Any characteristic that can be passed from parent to offspring.
Heredity - passing of traits from parent to offspring.
Genetics - The study of heredity.
Dominant - Uppercase in punnett square
Recessive Lowercase in punnett square
Allele - Alternate form of a gene
Homozygous / Purebred (RR,rr) - Two of the same alleles
Heterozygous / Hybrid (Rr) - One dominant, one recessive gene.
Phenotype - the physical trait of the gene
Gametes - Sperm or egg cell of a human, carries half of an offspring's DNA.
Foil method - First, outside, inside, last (used in punnett square of 4x4)
MONOHYBRID CROSS
’
DIHYBRID CROSSES
- A
NON-MENDELIAN GENETICS
Incomplete dominance
- The traits from each parents mixes together
- One allele is not fully dominant
Codominance
- Both traits from the parents will be express
Polygenic Traits
- A trait that is factor by many alleles
Epistasis
- When a trait needs needs another alleles to work
CHROMOSOMES & KARYOTYPES
Centromere
- Hold 2 chromatids together
Gene
- Segment of DNA that codes for a trait
Chromatids
- Identical copies
Chromosome Number
- All cells in the human body (SOMATIC CELLS) have 46 or 23 pairs of chromosomes
- Called the DIPLOID or 2n number.
- GAMETES (eggs & sperm) have only 23 chromosomes.
- Called the MONOPLOID or 1n number.
Nondisjunction
- Chromosomes may fail to separate during meiosis
- Resulting gametes may have too few or too many chromosomes
- Disorders
- Down Syndrome - three 21st chromosomes
- Turner Syndrome - single X chromosome
- Klinefelter’s Syndrome - XXY chromosomes
💀💀💀 FAILING TO SEPARATE IS CALLED NONDISJUNCTION 💀💀💀
NATURAL SELECTION
Stuff live and stuff don’t
Q3 EXAM REVIEW
- transcriptiondna structure,
- dna function,
- rna structure,
- rna function,
- mrna,
- trna,
- amino acids,
- codons,
- anticodons,
- central dogma,
- similarities and differences between dna and rna,
- monohybrid crosses,
- dihybrid crosses,
- meiosis,
- codominance,
- incomplete dominance,
- karotypes,
- multiple alleles,
- blood type,
- reading codon chart,
- mutations .
Questions amount
- 3 open endeds, 27 questions