Metallic properties and bonding
Metallic bonding = The electrostatic attraction between metal cations and a sea of delocalised electrons
metals are found on the left of the periodic table
all metals are found in s-subshells
Property | What this says about the structure |
Metals are dense and typically very heavy for their volume | high density |
Metals have high melting and boiling points | high melting and boiling points |
Metals conduct electricity in both molten and solid state | high electrical conductivity |
Metals are good conductors of heat | thermal conductivity |
Metals can be shaped and pulled (malleable and ductile) | malleable and ductile |
The metallic bonding model
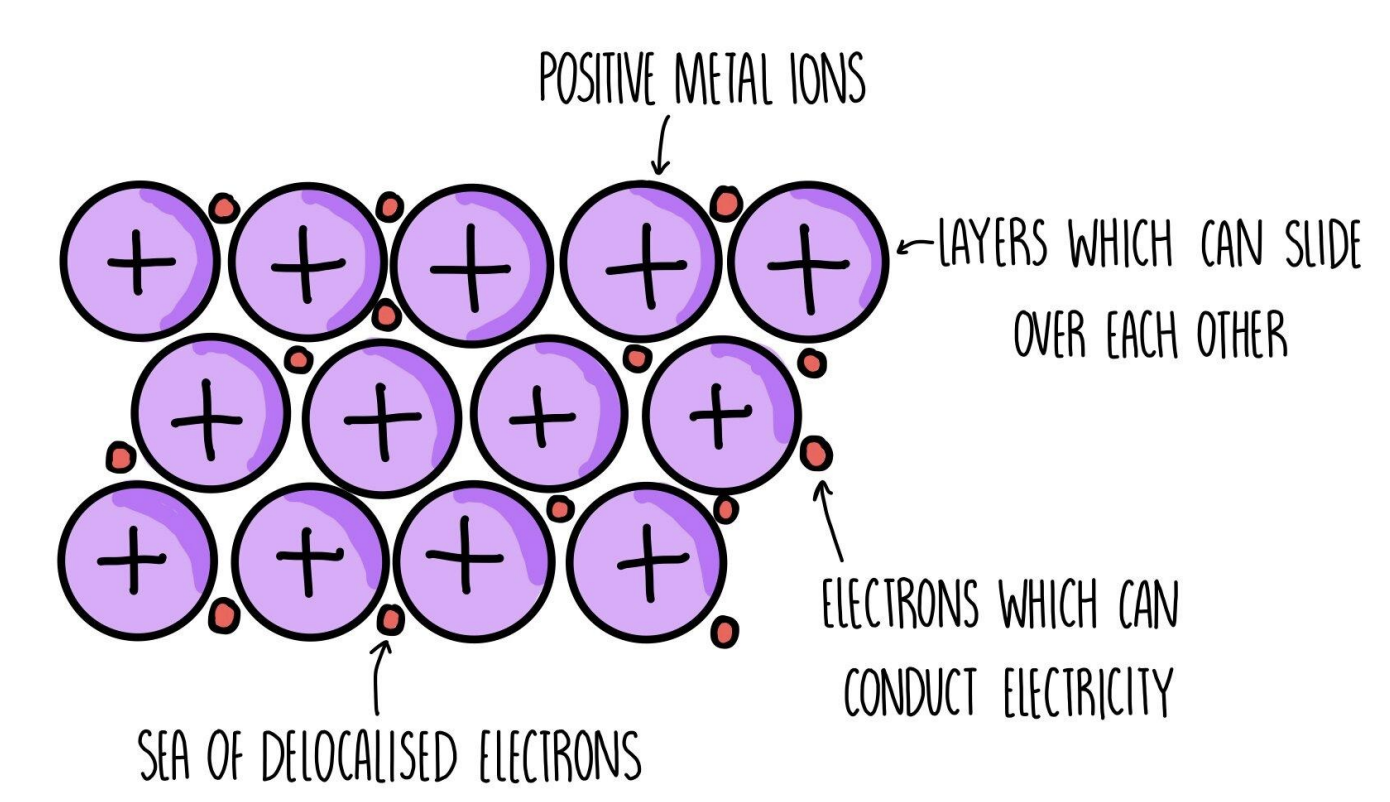
From atoms to metallic bonding
Remember that atoms are unstable if they don’t have a full outer shell and will gain/lose electrons to become full
The dots in the metallic bonding model are not nuclei, but ions (nuclei + inner electron shells)
two generalisations regarding the strength of metallic bonding and the type of metal atom involved can be made:
1. The strength of the metallic bonding in a metal INCREASES with decreasing atomic radius. Explain.
Smaller atoms → smaller ions → smaller distance between positive nucleus of ion and delocaised electrons → stronger electrostatic attraction between metal ions and delocalised electrons → stronger metallic bonding.
2. The strength of the metallic bonding in a metal INCREASES with increasing number of valence electrons that form the sea of electrons. Explain.
More valence electrons → greater charge on cation → stronger electrostatic attraction between metal ions and delocalised electrons → stronger metallic bonding.
Limitations to the model
Although this model of metallic bonding explains many properties, some cannot be explained this way
Properties which can’t be explained include:
Variation of melting points (Mercury melts at -39oC, Gallium at 30oC, iron at 1500oC, Tungsten at 3422oC)
Different densities, hardness of similar metals
Magnetic nature of iron, nickel, cobalt