Writing Chemical Formulae
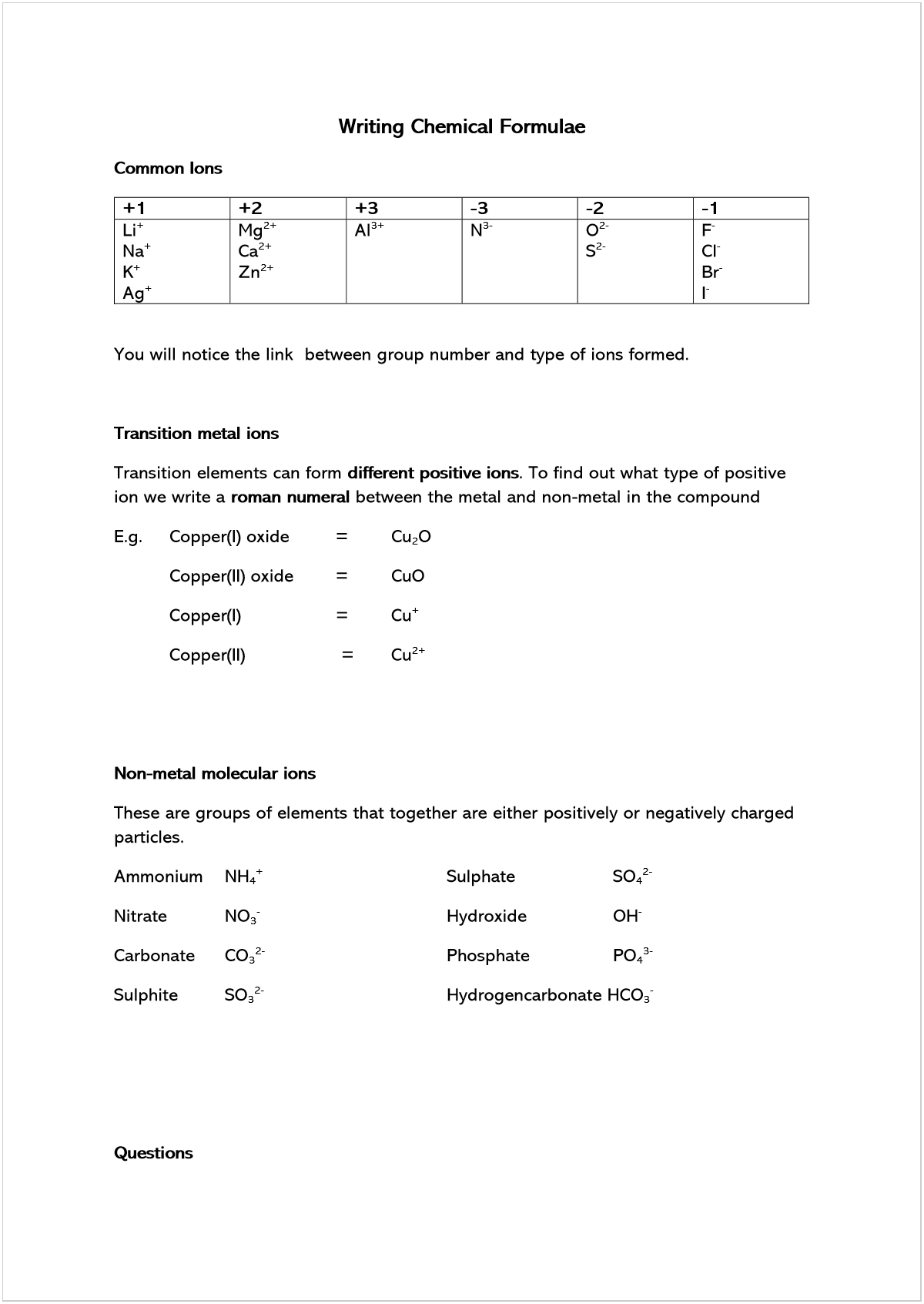
Common Ions
+1 +2 +3 -3 -2 -1
Li
+
Na
+
K
+
Ag
+
Mg
2+
Ca
2+
Zn
2+
Al
3+
N
3-
O
2-
S
2-
F
-
Cl
-
Br
-
I
-
You will notice the link between group number and type of ions formed.
Transition metal ions
Transition elements can form
different positive ions
. To find out what type of positive
ion we write a
roman numeral
between the metal and non-metal in the compound
E.g. Copper(I) oxide = Cu
2
O
Copper(II) oxide = CuO
Copper(I) = Cu
+
Copper(II) = Cu
2+
Non-metal molecular ions
These are groups of elements that together are either positively or negatively charged
particles.
Ammonium NH
4
+
Sulphate SO
4
2-
Nitrate NO
3
-
Hydroxide OH
-
Carbonate CO
3
2-
Phosphate PO
4
3-
Sulphite SO
3
2-
Hydrogencarbonate HCO
3
-
Questions