CIE A2 Biology 12.1: Energy in Living Organisms and ATP
Energy is required to carry out a variety of biological processes in living organisms and cells such as:
- active transport
- exocytosis
- movement
- anabolic reactions (synthesizing large molecules from smaller ones)
- homeostasis e.g maintaining body temperature
The sun is the primary energy source for nearly all organisms i.e plants. This happens in a process called photosynthesis.
- light energy is transformed into chemical potential energy
- photosynthesis produces 2,870 kJ of energy
- the carbohydrates produced in photosynthesis are then used to form ATP
- when energy from ATP is released, this is known as respiration
Features of ATP
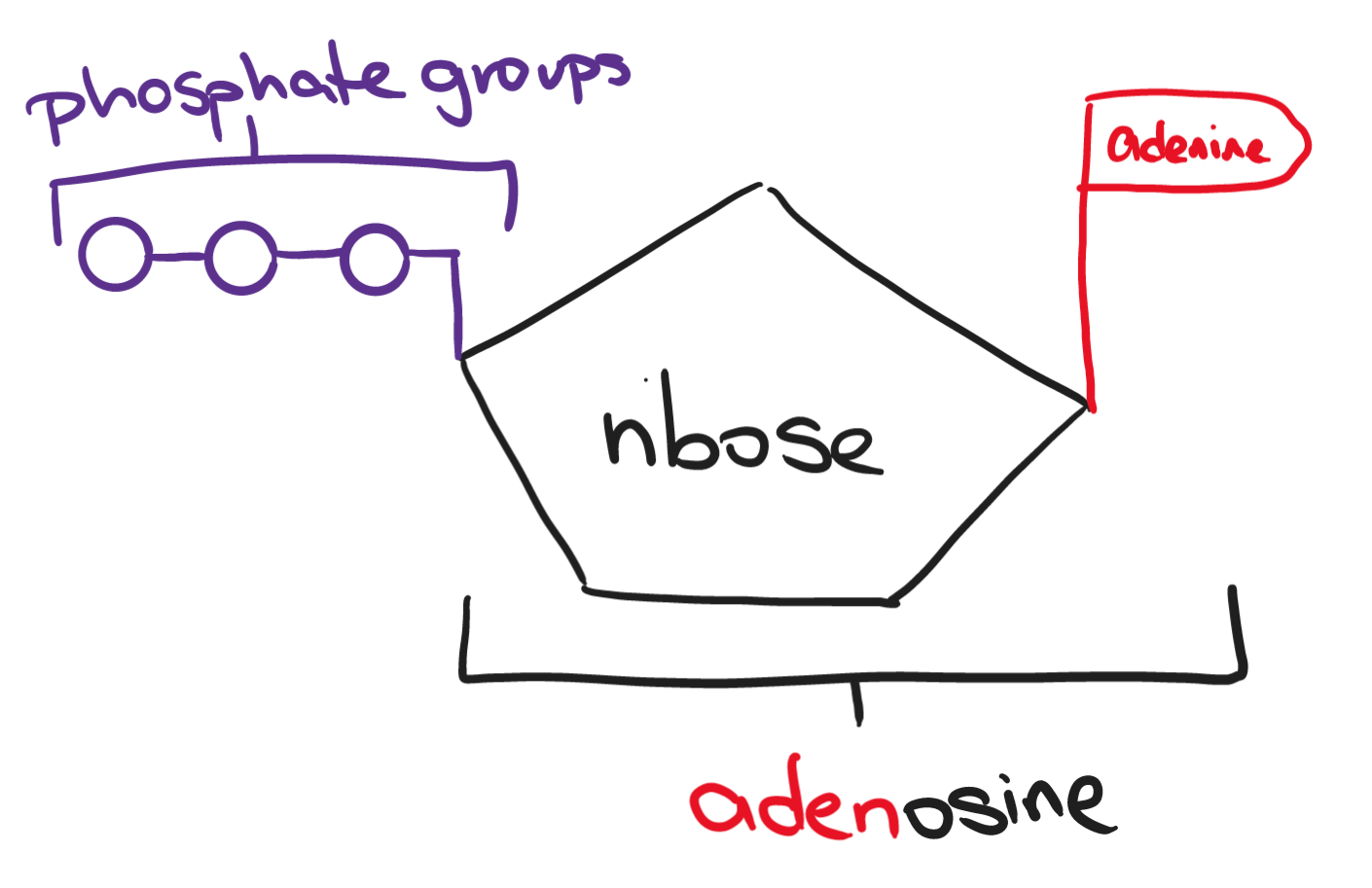
ATP (adenosine triphosphate) is the most known form of energy used. It is a small and soluble molecule that provides short-term energy. Each cell makes its own ATP and releases the energy from ATP molecules to carry out these processes. ATP is only made when the body needs it and does not store large amounts.
More features of ATP include:
- stable - ATPase is required to break it down so energy cannot be wasted
- recyclable - ADP and one phosphate group can make ATP and vice versa
- quick hydrolysis - energy can be released on demand
- soluble and moves easily within cells - can be used in other parts of the cell
- forms phosphorylated intermediates - makes metabolites more reactive and lower activation energy required for a reaction
Energy is released through the hydrolysis of ATP into ADP (adenosine diphosphate; produces 30.5 kJ/mol) and further into AMP (adenosine monophosphate; produces 30.5 kJ/mol again). Releasing the last phosphate and leaving only the adenosine produces only 14.2 kJ/mol of energy.
ATP is made by the human body through the addition of a phosphate group to ADP in respiration. This is done either by:
- a substrate-linked reaction where energy provided directly by another chemical reaction is used
- this reaction occurs in the cell cytoplasm and in the matric of mitochondria
- produces around 4-6 ATP per glucose molecule
- this reaction takes place in glycolysis
- chemiosmosis which takes place across the inner membranes of mitochondria and uses energy from the movement of hydrogen ions down their concentration gradient
- an electron transport chain establishes the proton concentration gradient; this releases energy
- produces around 32-34 ATP per glucose molecule and is the main form of ATP synthesis