High School Chemistry Unit 1
Electrons, Neutrons, Protons: Electrons are negatively charged subatomic particles orbiting the nucleus. Neutrons have no charge and are found in the nucleus. Protons are positively charged particles in the nucleus.
Atomic Number, Atomic Mass, Isotopes: Atomic number is the number of protons in an atom's nucleus. Atomic mass is the total mass of protons and neutrons. Isotopes are atoms of the same element with different numbers of neutrons.
Ions, Cations, Anions: Ions are charged atoms or molecules. Cations are positively charged ions, formed by losing electrons. Anions are negatively charged ions, formed by gaining electrons.
Calculating Ion Charge: To calculate ion charge, subtract the number of electrons from the number of protons for cations, or subtract the number of protons from the number of electrons for anions.
The mass number of an atom is the total number of protons and neutrons in its nucleus. It is represented by the symbol A. Mass number = number of protons + number of neutrons.
Absorption Spectrum: Shows absorbed wavelengths by a substance.
Emission Spectrum: Displays emitted wavelengths by a substance.
When Seen: Absorption spectrum when light passes through a substance, emission spectrum when substance emits light.
Atoms are way too small to see with the naked eye (and even most microscopes). So, we represent atoms using models.
Models help us visualize atomic structure. They also help us explain and predict the behavior of atoms.
However, it's important to remember that no scientific model is perfect. Every model sacrifices some accuracy for simplicity, visibility, or usability. If a model was perfect, it wouldn't be a model—it would be the real thing!
Atomic models are further complicated by quantum weirdness—electrons have both wave and particle properties.
Let's take a closer look at two atomic models, each with its own strengths and limitations.
The electron cloud model
An electron cloud model of a helium-4 atom is shown below.
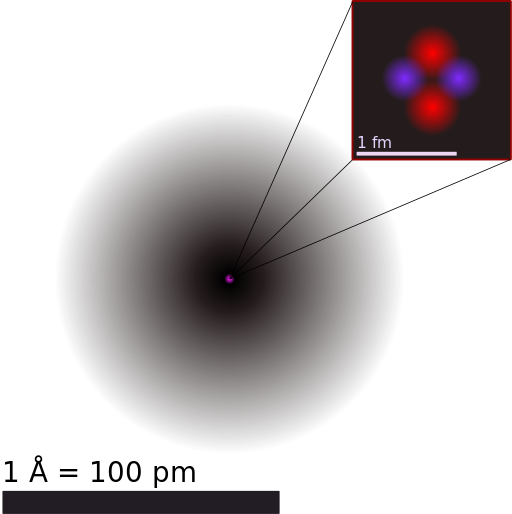
A model of a helium atom shows a black circle which fades to white moving from the center to the outside. At the center of the circle is a tiny nucleus, consisting of two red circles and two purple circles.
What do the scales mean on this model?
In this model, the black "cloud" represents the volume of space where electrons are likely to be found. The darker the region, the more likely electrons are to be found there.
The nucleus is shown as a tiny clump of red protons and purple neutrons in the center of the atom.
Strengths
A strength of this model is how it represents the wave behavior of electrons. The fuzzy electron cloud represents how individual electrons are actually spread out through space. Until we measure an electron's position, we don't know exactly where it is. The best we can do is describe where we're likely to find electrons around a nucleus. Quantum mechanics is weird!
Another strength of this model is how the nucleus is represented. We can see the individual protons and neutrons, represented in different colors. The nucleus is very small compared to the size of the electron cloud, which is true for real atoms. (Though the real nucleus is even smaller—it would be invisible if we drew it to scale on this model.)
Limitations
The fuzzy electron cloud does a good job representing the wave nature of electrons. However, the model doesn't show electron particles. From this model, we can't even tell how many electrons the atom has!
Since most of chemistry involves tracking what electrons are doing, it's often useful to use a another model which represents electrons in a different way.
The Bohr model
A Bohr model of a neutral helium-4 atom is shown below.
A model of a helium atom shows a green circle in the middle. Around the green circle is a gray ring. On top of the gray ring sit two black dots.
In this model, the electrons are represented as black dots that sit on a ring around the nucleus. The nucleus is shown as one green circle in the center.
Strengths
The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons.
As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr model represents these energy levels as rings. We can tell that the two electrons in the model above are at the same energy level because they are on the same ring.
Limitations
By representing electrons as particles, the Bohr model does not reflect the wave properties of electrons. The electrons appear to exist in specific locations, which is not entirely true.
Additionally, the nucleus in a Bohr model is typically shown as one circle, regardless of how many protons and neutrons are present in the nucleus.
How are electrons arranged in Bohr models?
Helium's Bohr model shows that the first two electrons are in the same energy level. But what about elements with more electrons?
It turns out that the first energy level holds a maximum of two electrons.
Beginning with lithium, a second energy level begins to fill with electrons. That second energy level can hold a maximum of eight electrons.
After the second energy level is filled with eight, a third energy level begins to fill.
Bohr models of some elements from the first three rows (periods) of the periodic table are shown below.
Bohr models of the following elements are shown, with the corresponding numbers of electrons (e), shown as dots, in each shell. H: 1 e in first shell He: 2 e in first shell Li: 2 e in first shell, 1 e in second shell Be: 2 e in first shell, 2 e in second shell O: 2 e in first shell, 6 e in second shell F: 2 e in first shell, 7 e in second shell Ne: 2 e in first shell, 8 e in second shell Na: 2 e in first shell, 8 e in second shell, 1 e in third shell Mg: 2 e in first shell, 8 e in second shell, 2 e in third shell S: 2 e in first shell, 8 e in second shell, 6 e in third shell Cl: 2 e in first shell, 8 e in second shell, 7 e in third shell Ar: 2 e in first shell, 8 e in second shell, 8 e in third shell
Why are some electrons paired?
Energy levels
The rings in a Bohr model represent the discrete energy levels that electrons can occupy. Electrons cannot exist at energies between these levels.
The energy levels in a Bohr model are also called shells. The shells are labelled as shown for the chlorine model below.
A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus is labelled n=1, the second ring from the nucleus is labelled n=2, and the third ring from the nucleus is labelled n=3.
The higher the shell number, the greater the energy of electrons in that shell. For example, electrons in the shell of the atom are at greater energy than electrons in the shell, which are at greater energy than electrons in the shell.
To summarize for the first two shells:
Energy level (shell) | Maximum number of electrons | Electron energy |
|---|---|---|
|
| lowest possible energy for the atom |
|
| greater energy than electrons in |
So, keep in mind that the shells of a Bohr model represent electrons' energy levels, NOT their positions or paths. Electrons do NOT move in circular paths around the nucleus.
Valence electrons
Electrons in the outermost shell of an atom are most easily transferred or shared with other atoms. So, an atom's outer electrons are usually the most important in chemistry.
The outermost shell of an atom is called the valence shell. Any electrons in the valence shell are called valence electrons.
Any electrons in an atom which are not in the valence shell are called core electrons.
In the chlorine model below, the valence electrons are shown in , and the core electrons are shown in .
A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus contains 2 black dots, the second ring from the nucleus contains 8 black dots, and the third ring from the nucleus contains 7 red dots.
So, we can tell from this model that chlorine has seven valence electrons.
Additional notes about Bohr models
Keep these things in mind when working with Bohr models:
The rings of a Bohr model do NOT represent circular paths followed by electrons. Electrons do NOT orbit the nucleus like planets orbit the sun. The rings are simply a convenient way to represent electron energy levels.
Sometimes, you may see a Bohr model with rings that get closer together as they get farther out. This represents how the difference between energy levels decreases with greater . However, since the rings are not intended to perfectly represent electron positions or energies, the exact spacing of the rings is not important.
Bohr models are not meant to represent what real atoms "look" like. In fact, real atoms are too small to look like anything! The particles inside them have no color and no definite shape. Everything is fuzzy and fluctuating.
The word "radiation" sometimes gets a bad rap. People often associate radiation with something dangerous or scary, without really knowing what it is. In reality, we're surrounded by radiation all the time.
Radiation is energy that travels through space (not just "outer space"—any space). Radiation can also interact with matter. How radiation interacts with matter depends on the type of radiation and the type of matter.
Radiation comes in many forms, one of which is electromagnetic radiation. Without electromagnetic radiation life on Earth would not be possible, nor would most modern technologies.

From space, the sun appears over Earth's horizon, illuminating the atmosphere as a blue layer above Earth. Above the atmosphere, space appears black.
Sunrise photo from the International Space Station. Earth's atmosphere scatters electromagnetic radiation from the sun, producing a bright sky during the day.
Let's take a closer look at this important and fascinating type of radiation.
Electromagnetic radiation
As the name suggests, electromagnetic (EM) radiation is energy transferred by electromagnetic fields oscillating through space.
EM radiation is strange—it has both wave and particle properties. Let's take a look at both.
Electromagnetic waves
An animated model of an EM wave is shown below.
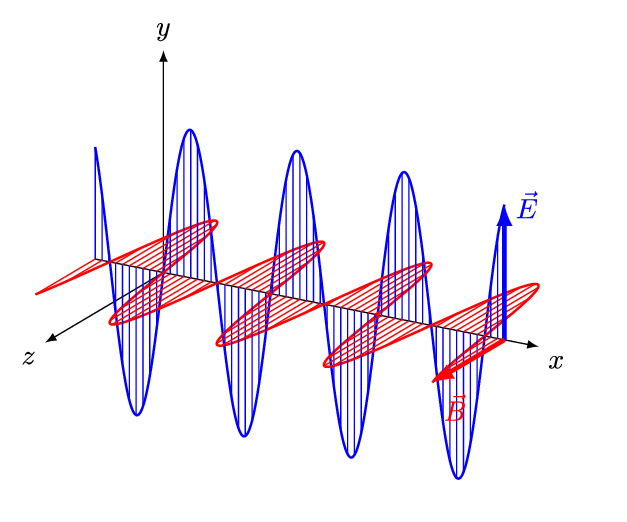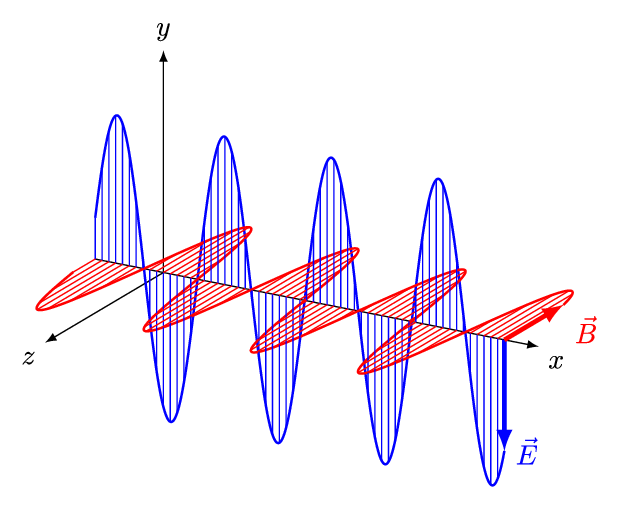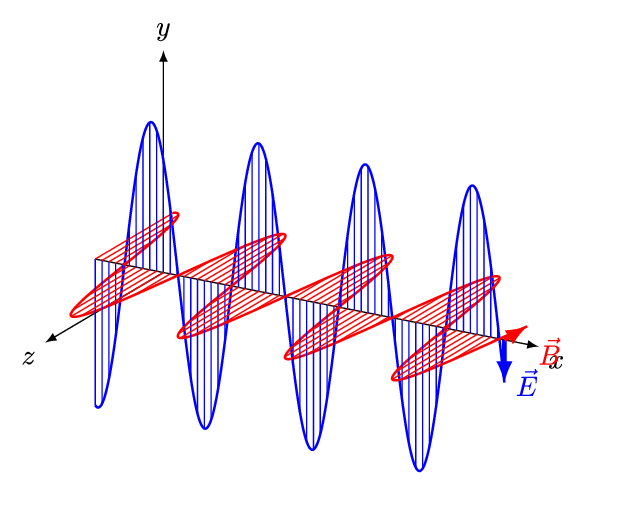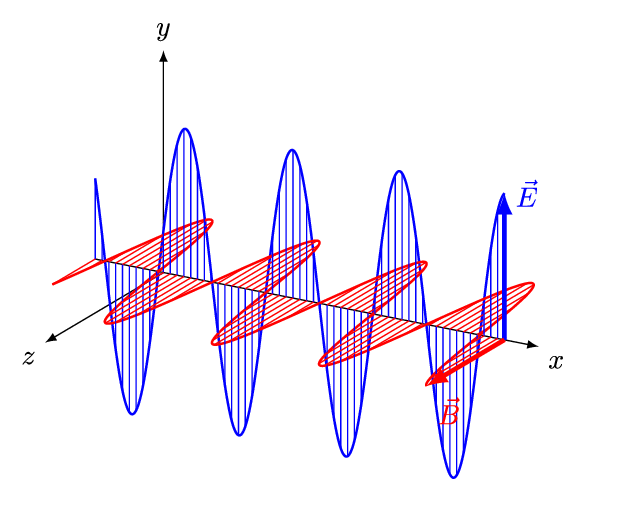
An animation shows a blue electric field arrow oscillating up and down. Connected to the base of the electric field arrow is a magnetic field arrow, which oscillates from side to side. The two fields oscillate in unison: when one extends the other extends too, creating a repeating wave pattern.
The electric field is shown in , and the magnetic field is shown in . They're perpendicular to each other.
A changing electric field creates a magnetic field, and a changing magnetic field creates an electric field. So, once the EM wave is generated it propagates itself through space!
As with any wave, EM waves have wavelength, frequency, and speed. The wave model of EM radiation works best on large scales. But what about the atomic scale?
Photons
At the quantum level, EM radiation exists as particles. A particle of EM radiation is called a photon.
We can think of photons as wave packets—tiny bundles of EM radiation containing specific amounts of energy. Photons are visually represented using the following symbol.
A squiggly curve drawn from left to right. The right end of the curve has an arrow point. The curve begins with a small amount of wiggle on the left, which grows in amplitude in the middle and then decreases again on the right. The result is a small wave packet.
All EM radiation, whether modeled as waves or photons, travels at the speed of light in a vacuum:
But, EM radiation travels at a slower speed in matter, such as water or glass.
The electromagnetic spectrum
The wavelength, frequency, and energy of EM radiation can fall within a wide range. We call that range the electromagnetic (EM) spectrum.
A diagram of the EM spectrum is shown below.
A diagram of the EM spectrum shows the following regions in order from lowest frequency and energy to highest frequency and energy. Each is accompanied by an object for comparison to its wavelength. Radio waves: wavelength on the scale of buildings Microwaves: wavelength on the scale of humans to butterflies Infrared: wavelength on the scale of a needle point Visible light: wavelength on the scale of protozoans Ultraviolet: wavelength on the scale of molecules X-rays: wavelength on the scale of atoms Gamma rays: wavelength on the scale of nuclei
Let's analyze one piece of this diagram at a time.
Wavelength
Wavelength is the distance from one peak of a wave to the next peak. Notice how the red wave at the top of the diagram shows the wavelength decreasing from left to right—the peaks get closer together.
The numbers on the diagram tell us that EM wavelength ranges from meters to meters.
Some EM waves have wavelengths longer than buildings while others are shorter than atoms. That's a big difference!
Frequency
Frequency is the number of wave cycles in a period of time. We can't "see" frequency in a stationary diagram, since frequency involves change over time.
But, we can visualize frequency with a simple example. Imagine you're creating water waves by dripping water into a pool. The more frequent the drips, the higher the frequency of waves which will spread out.

Water drips fall on a surface of water, creating circular waves that spread outward
If you release one drip per second, you'll create a wave with a frequency of hertz . If you release three drips per second, you'll create a hertz wave.
The numbers on the diagram tell us that EM frequency ranges from hertz to hertz.
Imagine that many water drips every second!
Energy
Finally, notice on the bottom of the EM spectrum diagram that photon energy increases as frequency increases.
The diagram does not show numerical values for energy, but the energy of EM radiation can be measured in various units.
One unit used to measure the energy of individual photons is the electronvolt .
To summarize for EM waves:
shorter wavelength higher frequency higher energy photons
Let's take a tour!
Each region of the EM spectrum has it's own name. Let's explore each region in more detail.
Radio waves
Radio waves are EM waves with the longest wavelength and lowest frequency. AM and FM radio signals are sent using radio waves.
Microwaves
Microwaves have higher frequency than radio waves. Microwaves are produced inside microwave ovens to warm food. Most WiFi signals are also in the microwave range.
Infrared
Infrared radiation has higher frequency than microwaves. Objects at "everyday" temperatures we interact with regularly, including our bodies, radiate infrared waves. So, we commonly associate infrared radiation with warmth.
Infrared cameras show the amount of infrared radiating from an area. For example, this infrared image of Rusty the dog shows that the greatest amount of infrared radiation escapes from his eyes, mouth, and ears. His fur traps much of the energy on the rest of his body, keeping him warm.
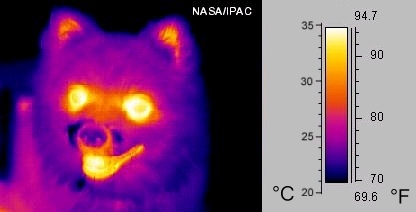
An infrared image of a dog's face, represented in visible colors. Yellow regions emit more infrared, and purple regions emit less infrared. In the infrared image, the dog's open mouth, eyes, and ears are yellow, and the rest of his face is purple.
Infrared waves also radiate from surfaces warmed by the sun, like soil and concrete. Some of that infrared radiation escapes into space, and some is trapped by Earth's atmosphere.
Visible light
The next highest frequency band is visible light. This band of EM radiation is called "visible" because it's what human eyes can see.
Sighted people can only see when visible light enters their eyes. Nothing is visible in a room without visible light.
Our brains perceive different frequencies in the visible spectrum as different colors. We see the longest wavelength, lowest frequency visible light as , and the shortest wavelength, highest frequency visible light as .
All the other colors of the are associated with frequencies between red and violet. Other colors, like white, result from mixtures of these frequencies entering our eyes at the same time.
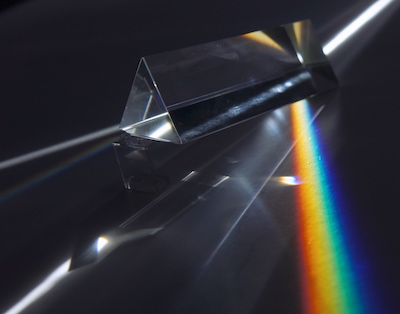
A triangular glass prism spreads out different wavelengths of visible light. A beam of white light enters the prism on one side, and a rainbow of colors exits the other side.
Does "light" only refer to visible light?
Ultraviolet (UV)
As EM frequency increases beyond violet light, we lose the ability to see it with our eyes. We've entered the ultraviolet—or UV—range.
Remember that photon energy increases with frequency. The energy of UV photons is high enough that they can cause damage to living tissues.
In addition to visible light, UV radiation enters Earth's atmosphere from the sun. The ozone layer blocks much of it, but some UV makes it to Earth's surface.
So when you're outside in the sun, make sure that you use UV protection to avoid sunburns and eye damage.
X-rays
As EM frequency increases beyond UV, we arrive at X-rays.
The energy of X-ray photons is high enough to make it ionizing radiation. This means that if an X-ray photon enters matter, it can knock electrons out of (ionize) atoms in the matter.
This is dangerous for organisms, since repeated ionizations in cells can lead to damage and mutations.
However, the small dose of X-ray radiation received during a medical scan is safe. If you've ever had an X-ray image taken of your body, X-ray photons were sent into you. Dense tissues like bone block more X-rays than soft tissues, creating a shadow image when the X-rays reach a detector on the other side.
Can you tell what's wrong with this person's arm from the X-ray image? Ouch.
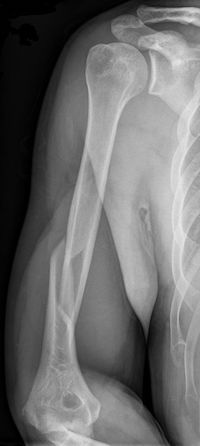
A black and white X-ray image of a person's arm shows the arm bone in white, and the surrounding tissues in lighter gray. The bone is fractured near the elbow.
Gamma rays
Finally, we reach gamma rays. Gamma radiation has the highest frequency and energy of any EM radiation. Like X-rays, gamma rays are ionizing radiation. They're the most hazardous to living things.
Gamma rays are produced by high energy interactions in space. Gamma rays are also produced by unstable nuclei during a type of radioactive decay called gamma decay.
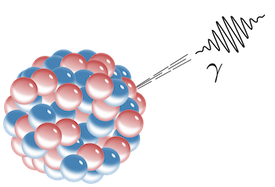
A large nucleus is shown as a clump of red protons and blue neutrons. A gamma photon, shown as a squiggly wave packet, shoots out of the nucleus.
Though gamma rays are hazardous, they can also be used for good. For example, gamma rays are used in radiation therapy to kill cancer cells.
Summary
Electromagnetic radiation consists of oscillating electric and magnetic fields. It can be modeled both as waves and as particles called photons. All EM radiation, from radio to gamma, travels at speed in a vacuum. The higher the frequency of EM radiation, the higher the energy of the photons.
Human eyes can only see the visible light portion of the spectrum directly. However, we can use various detectors to make the other regions of the EM spectrum visible to us.
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in .
A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus contains 2 black dots, the second ring from the nucleus contains 8 black dots, and the third ring from the nucleus contains 7 red dots.
The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have.
Fortunately, we can use the periodic table to quickly determine the number of valence electrons for main group elements.
Valence electrons on the periodic table
Bohr models for the first three periods of the periodic table are shown below. The valence electrons are shown in .
Do you notice any patterns in the number of valence electrons?
Bohr models of the first 18 elements are shown, arranged in rows and columns. The number of valence electrons increases from left to right across a row. The number of valence electrons is the same going down a group.
The number of valence electrons increases going left to right across a period. But, with the exception of , the number of valence electrons remains the same going down a group.
For example, , , and are in group 1 and all have one valence electron. Both and are in group 15 and have five valence electrons.
This is why elements in the same group have similar chemical properties—they have the same number of valence electrons!
This pattern allows us to quickly identify the number of valence electrons for main group elements on the periodic table, even if we don't have Bohr models to reference. The diagram below shows the number of valence electrons (VE) for the main group elements.
Lewis Diagrams
Lewis diagrams, also known as Lewis structures, represent the valence electrons of atoms in a molecule. Each dot or line represents a valence electron. For example, in H2O, oxygen has 6 valence electrons and hydrogen has 1, resulting in a diagram with O at the center surrounded by two H atoms, each sharing a pair of electrons with O.
Atomic Radii Trends: How different sizes of atoms relate to each other. Atoms are not all the same size. The relative size of the atoms follows a set of trends on the periodic table. Going across a period, the main group elements tend to decrease in atomic radius due to the increased nuclear charge. Going down a group, the main group elements tend to increase in atomic radius due to increased shielding and the addition of new shells to the structure of the atom. When moving down a group, you’re adding more protons, but you’re also adding more electrons, filling up the lower shells and forcing the electrons into higher energy shells that stay further away from the nucleus. When you move right across a period, you’re adding more protons and electrons, but you stay at the same number of shells, rather than forcing electrons into higher shells, allowing the magnetic attraction between the protons and electrons to keep the radius of the atom smaller.
Ionization is the process of removing an electron from a neutral atom (or compound). The energy required to remove an electron is the ionization energy. The ionization energy differs for each atom. There are trends that match the structure of the periodic table. Across a period, ionization energy tends to increase. Down a group, the ionization energy tends to decrease.
Across a period, ionization energy tends to increase due to increasing nuclear charge and effective nuclear charge.
Down a group, ionization energy tends to decrease because of increased atomic size and shielding effect.
Atoms form stable ions by gaining or losing electrons until they have a full valence shell.
There are two possible ways this can occur:
Nonmetals gain electrons until there are eight in the valence shell.
Metals lose the electrons in their existing valence shell, leaving the full shell below as the new valence shell.
We can use the periodic table to determine that sulfur is a nonmetal. Therefore, it will gain electrons to form an ion.
The Lewis diagram shows us that sulfur has six valence electrons. This means it needs to gain two electrons to fill its valence shell.
When a neutral atom gains two electrons, it forms an ion with -2 charge.
So far, we've considered elements that typically form cations of one particular charge. For example, the alkali metals and the alkaline earth metals usually form 1+ ions and 2+ ions, respectively.
Most transition metals and several metals in groups 13-17, however, can form cations of various charges. For instance, iron is often found as both the and cations, and sometimes other charges as well. Thus, iron is polyvalent, which literally means "many valued"—it is able to form cations of different charges.
For metals that are polyvalent, we need to specify the magnitude of the charge on the ion. We do this by adding Roman numerals to the name, which indicate the charge of the cation. For example, is written as "iron(II)", and is written as "iron(III)."
In speech, we have to call "iron two-plus" or "iron two" because simply referring to it as an "iron ion" will not give enough information to specify the cation charge.
Cations are positively charged ions formed when neutral atoms lose electrons; anions are negatively charged ions formed when neutral atoms gain electrons. It is possible to predict the charges of main group ions by looking at the group numbers on the periodic table.
To name main group cations, we simply refer to them as the element's name + "ion". To name main group anions, we add the suffix -ide to the end of the element's name.
Cations and anions combine to form ionic compounds. Ionic compounds are named with the cation first and the anion last. The same convention is used when writing their chemical formulas. Ionic compounds must be electrically neutral. Therefore, the cations and anions combine in such a way that the net charge contributed by the total number of cations exactly cancels the net charge contributed by the total number of anions.
Naming cations typically involves using the name of the element followed by the word "ion" or simply the name of the element itself. Unlike anions, cations do not have a specific suffix like "-ide." They are named based on the name of the element from which they are derived. For example:
Sodium cation: Na⁺
Calcium cation: Ca²⁺
Aluminum cation: Al³⁺
So, for cations, the naming convention is simpler compared to anions, and no suffix like "-ide" is used.
In chemistry, compounds ending in "-ide" typically indicate binary compounds, while those ending in "-ine" often refer to polyatomic ions or organic compounds.
Covalent bonds involve shared electron pairs between atoms. Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the shared pair. Atoms share the same number of pairs needed to fill their valence shell, usually with eight. Hydrogen only needs one additional electron to fill its valence shell, so it shares only one pair.







