IB Chemistry SL Organic Lesson 6: Alcohols
Alcohols as Fuels
Alcohols combust more readily (break bonds - lower bond enthalpy) than equivalent alkanes but release less energy since they are already partially oxidized.
Alcohol + oxygen → carbon dioxide + water
Alcohols are used a fuels:
As a fuel for cars - either pure or blended with petrol.
Methanol as fuel for competitive motor-sports including dragsters and monster trucks.
Much fuel ethanol is fermented from crops… crops that could otherwise be eaten, forcing up food prices. Is this ok?
Grain + yeast → alcohol (ethanol)
 Oxidation state of carbon:
Oxidation state of carbon:
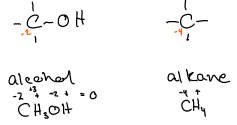 Carbon in alcohol (methanol) is -2. Carbon is the alkane (methane) is -4.
Carbon in alcohol (methanol) is -2. Carbon is the alkane (methane) is -4.
CH4 → CO2 (Has a movement of 8 electrons - carbons oxidation #)
CH3OH → CO2 (Has a movement of 6 electrons - release less energy)
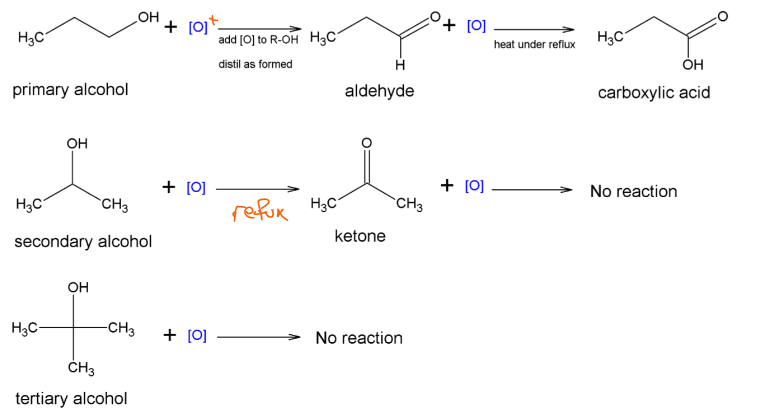
Oxidation of alcohols
The most important reactions of the alcohols are their oxidation’s.
A range of compounds will oxidize them so the oxidizer is often represented as [O].
One oxidizing agent you need to know is potassium dichromate K2Cr2O7.
When using this, orange Cr (VI) is reduced to green Cr (III).
C2H5OH + Cr2O7-2 → CH3COOH + Cr3+
oxidation 3x(H2O + C2H5OH → CH3COOH + 4H+ + 4e-)
reduction 2x(6e- + 14H+ + Cr2O7-2 → 2Cr3+ + 7H2O)
16H+ + 2Cr2O7-2 + 3C2H5OH → 11H2O + 3CH3COOH + 4Cr+3
Oxidation Reaction Schemes