Stereoisomerism and Isomers
10.5 Stereoisomerism (HL)
Types of Stereoisomerism
Structural isomers have the same molecular formula but different atomic connectivity (as seen on page 441). Stereoisomers, on the other hand, possess the same structural formula (same connectivity) but differ in the arrangement of atoms in space.
Isomers Diagram
Isomers are categorized as structural or stereoisomers. Stereoisomers are further classified as configurational or conformational isomers(Figure 10.86).
ISOMERS
├── STRUCTURAL ISOMERS
└── STEREOISOMERS
├── CONFIGURATIONAL ISOMERS
│ ├── cis-trans ISOMERS
│ └── OPTICAL ISOMERS
└── CONFORMATIONAL ISOMERS
Learning Objectives
Understanding stereoisomerism.
Recognizing cis-trans or E/Z isomers in alkenes and cycloalkanes.
Explaining optical isomerism.
Determining if a molecule exhibits optical isomerism.
Using a polarimeter to distinguish optical isomers.
Defining a racemic mixture.
Understanding conformational isomerism.
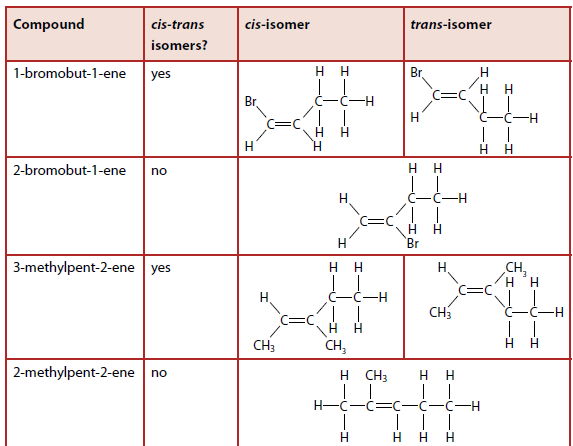
cis-trans Isomerism
cis-trans isomerism occurs in alkenes and cyclic compounds. The term 'geometric isomerism' is discouraged by IUPAC.
cis-trans isomerism: compounds with the same structural formula but different arrangements of groups around a double bond or a ring.
Double Bond Nature
A double bond consists of sigma (\sigma) and pi (\pi) components (Figure 10.87).
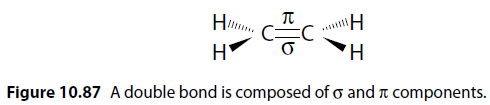
Rotation
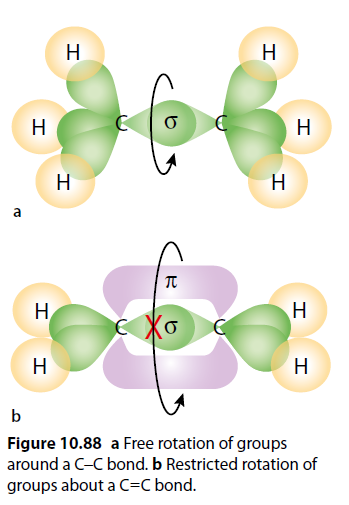
Rotation is possible around a C-C single bond due to the nature of the sigma bond. However, the pi component in a C=C bond restricts rotation (Figure 10.88). Breaking the pi bond requires significant energy.
Examples of cis-trans Isomerism
The restricted rotation around a double bond gives rise to cis-trans isomerism.
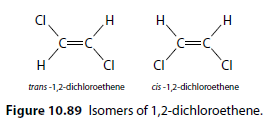
1,2-dichloroethene: The chlorine atoms are either on the same side (cis) or opposite sides (trans) of the double bond (Figure 10.89).
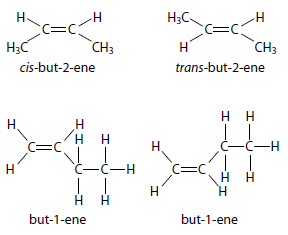
but-2-ene: Exhibits cis-trans isomerism, while but-1-ene does not (Figure 10.90).
Condition for cis-trans Isomerism
For a molecule to exhibit cis-trans isomerism, there must be two different groups on both sides of the double bond.
Examples Table
Table 10.22 shows examples of molecules that do and do not exhibit cis-trans isomerism.
cis: same side
trans: opposite sides
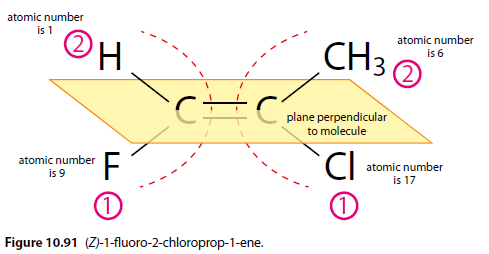
E/Z Naming System
The cis and trans nomenclature has limited value when there are four different groups around the C=C bond. The E/Z naming system is a more general way of naming isomers.
Cahn-Ingold-Prelog (CIP) Priority Rules
Each group attached to the C=C bond is given a priority based on the Cahn-Ingold-Prelog priority rules. Higher priority is given to atoms with a higher atomic number.
E/Z Designation
If the two highest priority groups are on the same side of a plane perpendicular to the double bond, the isomer is labeled 'Z' (zusammen - together). If they are on opposite sides, it is labeled 'E' (entgegen - opposite).
Example
(Z)-1-fluoro-2-chloroprop-1-ene (Figure 10.91).
Priority of Larger Groups
With larger groups, continue along the chain until a difference is found. For example, CH2CH3 has a higher priority thanCH_3.
Double-Bonded Atoms
Double-bonded atoms count as two groups attached to an atom. -CHO has higher priority than -CH_2OH.
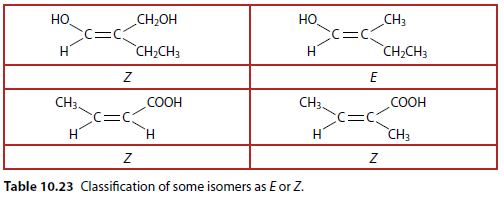
E/Z Isomers Table
Table 10.23 shows some molecules classified as E or Z isomers.
Properties of cis-trans Isomers
cis-trans (E/Z) isomers have different physical properties (Table 10.24).
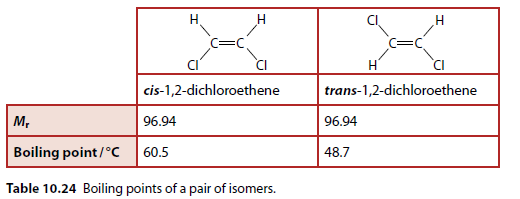
The structures of cis-1,2-dichloroethene and trans-1,2-dichloroethene differ in the orientation of their chlorine atoms (Figure 10.94). This leads to the cis form being polar and the trans form being non-polar.
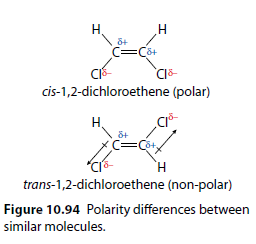
The trans form is non-polar because the dipoles cancel out. The cis form has permanent dipole-dipole interactions. Both molecules have similar London forces, so the difference in boiling points is due to the permanent dipole-dipole interactions.
cis-trans (E/Z) isomers may also have different chemical properties.
cis-trans Isomerism in Substituted Cycloalkanes
A cycloalkane is a ring compound containing only single C-C bonds and hydrogen. Cycloalkanes are structural isomers of the corresponding alkene.
Ring Compounds
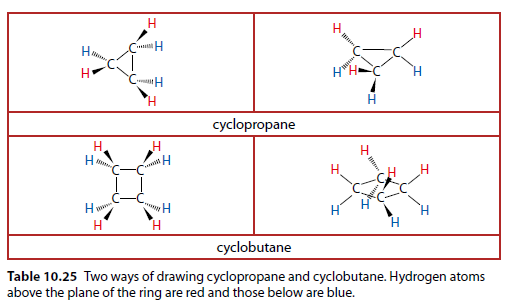
Table 10.25 shows two ways of drawing cyclopropane (C3H6) and cyclobutane (C4H8).
Restriction of Rotation
The ring structure prevents rotation, making cis-trans isomers possible in substituted cycloalkanes. Rotation requires breaking the ring, which needs a lot of energy.
Condition for Cycloalkane Isomerism
At least two carbon atoms must have two different groups attached.
Example: 1,2-dimethylcyclobutane
Table 10.26 shows structures in different ways, with colors indicating the convention used in Table 10.25.
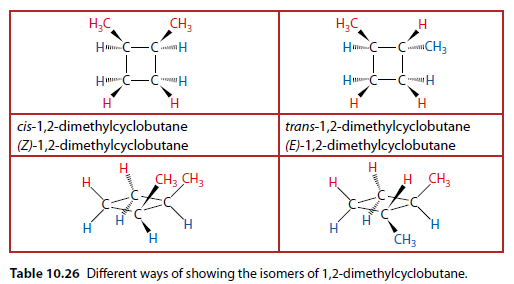
The E/Z nomenclature is used similarly to alkenes.
Note
1,1-dimethylcyclobutane is a structural isomer of 1,2-dimethylcyclobutane and does not show cis-trans isomerism.
1,3-dimethylcylobutane is a structural isomer of 1,1-dimethylcyclobutane and 1,2-dimethylcyclobutane and does exhibit cis-trans isomerism.
Conformational Isomers
Conformational isomers are forms of the same molecule with different conformations due to rotation about a sigma (\sigma ) bond. For example, 1,2-dichloroethane (Figure 10.95).
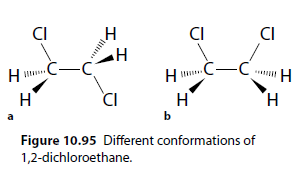
Molecule 10.95a has lower potential energy than molecule 10.95b because the chlorine atoms are further apart. There is a low barrier to rotation between forms.
Figures 10.95 and 10.96 represent the same molecule, and individual conformational forms cannot be isolated because the barrier to rotation is low. Different forms exist simultaneously and interconvert constantly.
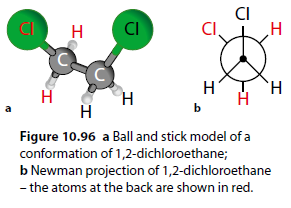
The rate of interconversion depends on the barrier to rotation and the temperature.
A common example is the chair and boat forms of cyclohexane (C6H{12}) (Figure 10.97).
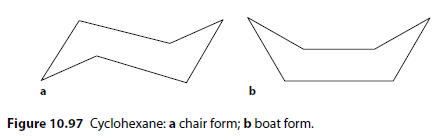
Key Differences
Conformational isomers can be interconverted without breaking chemical bonds.
Configurational isomers require breaking and re-forming chemical bonds.
Optical Isomerism
Optical isomerism is another type of stereoisomerism. Butan-2-ol exhibits optical isomerism. There are two forms that are mirror images (Figure 10.98).
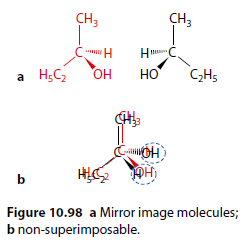
Non-Superimposable Mirror Images
Although they look identical, they are non-superimposable (Figure 10.98b).
Condition for Optical Isomerism
There must be four different groups attached to a carbon atom.
Butane (Figure 10.99) does not exhibit optical isomerism because it has a maximum of only three different groups attached to any one carbon atom, and the mirror images are superimposable.
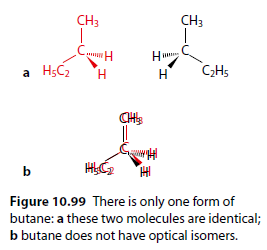
Chiral Centre
A carbon atom with four different groups attached is called a chiral centre (chirality centre or stereogenic unit). Molecules that exhibit optical isomerism are often described as chiral. The configuration of the chiral centre refers to the spatial arrangement of groups.
Examples Table
Table 10.27 shows molecules that exhibit optical isomerism. The chiral centers are shown in red.
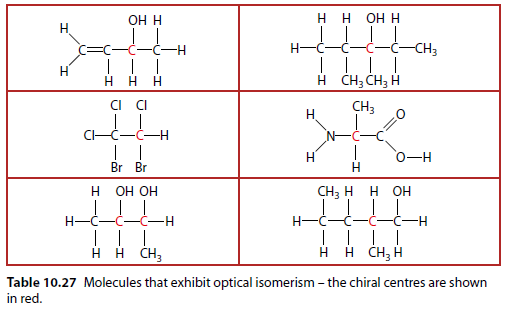
Asymmetric Carbon Atom
A carbon atom with four different atoms or groups attached is sometimes called an asymmetric carbon atom.
Molecules That Do Not Exhibit Optical Isomerism
Table 10.28 shows molecules that do not exhibit optical isomerism.
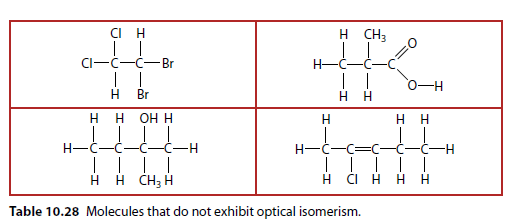
Tetrahedral Structure
The fact that these carbon compounds exhibit optical isomerism allows us to deduce that these molecules are tetrahedral.
Three-Dimensional Representation
Optical isomers are usually drawn in three dimensions (Table 10.28). A solid wedge indicates a bond coming out of the plane, and a dashed wedge goes into the paper.
Enantiomers
The individual optical isomers of a compound are called enantiomers.
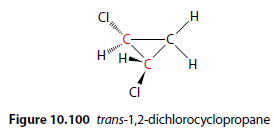
Optical Isomerism and Ring Compounds
Optical isomerism can also occur with ring compounds. trans-1,2-dichlorocyclopropane has two chiral centres (Figure 10.100).
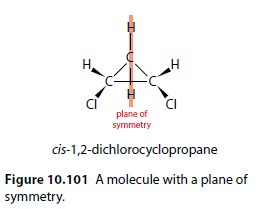
Plane of Symmetry
To determine if a ring compound has optical isomers, check for a plane of symmetry. If a molecule has a plane of symmetry, it will not have optical isomers. cis-1,2-dichlorocyclopropane has a plane of symmetry and does not exhibit optical isomerism (Figure 10.101).
Chirality in Ring Structures
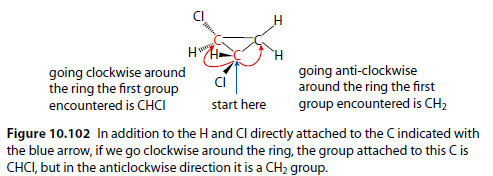
Figure 10.102 shows how to determine if a ring carbon atom has four different groups attached by going around the ring in two directions.
Ring Compounds Examples Table
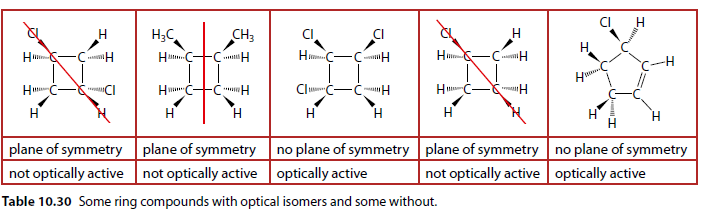
Table 10.30 shows some ring compounds with and without optical isomers.
Optical Isomers and Plane-Polarised Light
The two enantiomers of an optically active compound rotate plane-polarised light in opposite directions.
Plane-Polarised Light
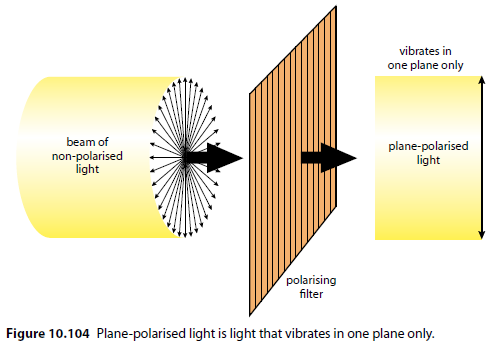
Normal, non-polarised light vibrates in all planes (Figure 10.103). Passing non-polarised light through a polarising filter produces plane-polarised light (Figure 10.104).
Optical isomers rotate the plane of plane-polarised light in opposite directions.
Polarimeter
A simple polarimeter consists of a light source, two polarising filters, a sample tube, and a scale to measure the rotation (Figure 10.105).
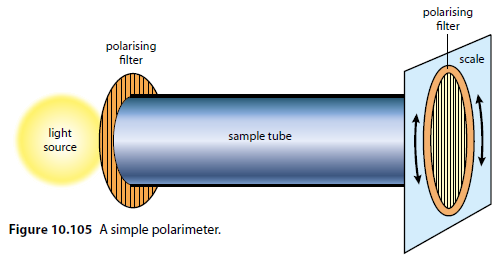
Determining Rotation
The solvent is placed in the sample tube, and the second polarising filter is rotated until no light is seen. The sample is then added, and the filter is rotated again. The difference in readings gives the angle of rotation.
Optical isomers can be distinguished using a polarimeter.
Racemic Mixtures
An equimolar mixture of two enantiomers is called a racemic mixture (or racemate). It has no effect on plane-polarised light because the rotations cancel out.
Formation of Racemic Mixtures
Reactions producing chiral carbon atoms usually produce a racemic mixture. A stereospecific synthesis is needed to make one specific enantiomer.
SN2 Reaction
An example is the \text{S}_N2 nucleophilic substitution reaction of 2-bromobutane with aqueous sodium hydroxide (Figure 10.107). Carried out with one enantiomer, the product will be the inverted enantiomer.
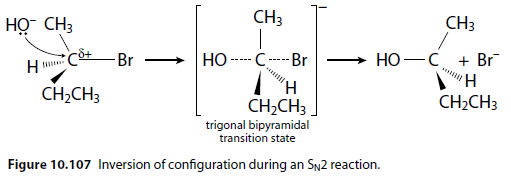
The reaction occurs with inversion of the configuration at the chiral centre.
The \text{S}_N1 mechanism is not stereospecific and produces a racemic mixture if the product has a chiral centre.
Diastereomers
Diastereomers are stereoisomers that are not mirror images of each other (e.g., cis-trans isomers). They also arise when more than one chiral centre is present.
Figures 10.108 and 10.109 show examples of enantiomers and diastereomers.
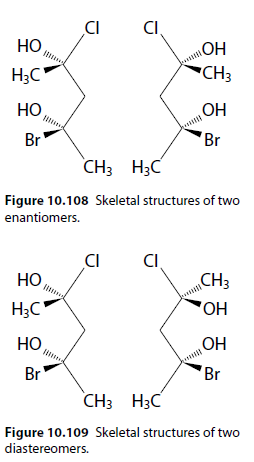
Properties
Physical properties are identical in enantiomers but not in diastereomers.
Chemical properties are identical for enantiomers reacting with non-optically active compounds. Diastereomers may have different chemical properties.
Thalidomide Example
The sedative thalidomide, given as a racemic mixture, had one enantiomer (S-enantiomer) responsible for teratogenic effects (Figure 10.110).
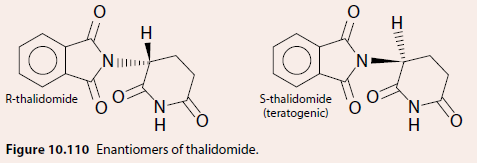
The enantiomers interconvert in the body, producing the racemic mixture. If a new drug is going to be marketed as the racemic mixture, testing must be carried out on each enantiomer separately and also on the racemic mixture.
Pharmaceutical companies now tend to synthesize or separate out the active single enantiomer of a drug and develop this instead of the racemic mixture. Different countries have different standards for approval of drugs - thalidomide was not licensed for sale in the US.
Nature of Science
The three-dimensional shape of a molecule and stereochemistry are extremely important to reactions going on in the body and a knowledge of this is critical in some areas of biochemistry and other biological sciences.