Factors affecting the rate of diffusion
Surface area: The larger the surface area over which diffusion can take place, the faster the rate of diffusion.
Distance: The greater the distance the longer the particles take to move randomly down the concentration gradient and the slower the rate of diffusion.
Concentration gradient: The greater the difference in concentration between the two sides of the membrane (the steeper the concentration gradient), the faster the rate of diffusion.
Temperature: The higher the temperature, the more kinetic energy the particles have, so they move faster and the faster the rate of diffusion.
Size of the particles: Larger molecules move more slowly and do not fit as easily between the phospholipids, so the slower the rate of diffusion.
Type of particles: Large, polar or charged molecules and ions require a transport protein to cross the membrane. Therefore the more transport proteins in the membrane the faster the rate of diffusion.
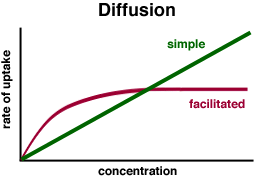
For simple diffusion, the rate of uptake is directly proportional to the difference in concentration either side of the membrane.
For facilitated diffusion, the rate of uptake plateaus when the number of transport proteins becomes limiting.