Biology test review
8.1
metabolism- refers to all of the chemical reactions (anabolic and catabolic) that occur in a living organism
metabolic pathway- linked series of chemical reactions occurring within a cell
these are enzyme meditated, usually requiring a different enzyme at each step or stage
Induced fit- when an enzyme takes in the substrates it changes its shape slightly to either encourage bonds to form (anabolic) to break them apart (catabolic)
substrate specificity- enzymes have specific substrates
enzyme inhibitor- blocks the function of enzymes (competitive and non-competitive)
competitive inhibitors- interact with the active site. more substrate is needed to counter act the effect of the inhibitor
non-competitive inhibitors- interact with a secondary site (allosteric site), this interaction changes the overall shape of the enzyme thus blocking the active site.
statins- a group of medicines that can help lower the level of density lipoprotein (LDL) cholesterol in the blood.
it’s the bad cholesterol and statins reduce the production of it inside the liver
they are a competitive inhibitor of enzymes that produced cholesterol, this inhibition is temporary
penicillin
background: bacteria walls are made of peptidoglycan
the enzyme transpeptidase builds these walls by linking peptides together
the antibiotic penicillin irreversibly binds to and permanently inhibits the activity of transpeptidase enzyme (non-competitive)
as a result the cell walls cannot be made and the bacteria die
end-product-inhibition
is used to regulate metabolic pathways
the end product can bind to an allosteric state of the first enzyme in the pathway which slows the processes.
ex: threonine is made into isoleucine, if too much isoleucine is made it will bind to the first enzyme to slow the process.
Cellular respiration
cell respiration- controlled release of energy from organic compounds to produce ATP
Aerobic vs. Anaerobic Respiration
energy is neither created nor destroyed, energy is transferred or converted
oxidation and reduction- this involves the transfer of electrons from one substance to another. this electron transfer also transfers potential energy
oxidation results in lower potential energy. (red-higher potential energy)
electron carriers- can carry and give up electrons (therefore energy). they usually link oxidation and reduction in cells. NAD is the primary electron carrier.
NAD to NADH is reduction
anaerobic respiration (fermentation)- allows for glycolysis- splitting of simple sugars (usually glucose) making a small amount of ATP
the split is into two molecules called pyruvate
2 ATP molecules are produced
pyruvate is further processed to release more energy
this ATP yield is enough for small organisms like yeast and bacteria
Lactic acid Fermentation
is in some animals, bacteria and some fungi
makes a small amount of ATP
makes lactic acid (lactate) as a waste product
humans experience this when exercising due to oxygen debt
Alcohol Fermentation
anaerobic
in fungi, yeast, and some bacteria
small yield of ATP
produces ethanol (booze) and CO2 (bread)
waste= CO2 ethanol
Glycolysis- small net gain of ATP without oxygen
step 1- Phosphorylation (addition of P) (glucose to hexose biphosphate)
this required 2 ATP molecules
this generate a hexose biphosphate molecule, this is unstable and allows the sugar to split easily
makes molecules less stable therefore more reactive
step 2- lysis (hexose biphosphate to 2 triose phosphate)
splits the hexose bisphosphate into 2 triose phosphate molecules
step 3- oxidation (is loss of electrons)
involves another phosphorylation but does not require ATP. Inorganic phosphate Pi is added. two triose biphosphate molecules are produced. NAD is reduced to NADH + H.
step 4- ATP formation (2 pyruvate)
involves the transfer of phosphate from each triose bisphosphate molecule to molecules of ADP. 4 ATP molecules are formed from the 4 Phosphate groups. (2 molecules of pyruvate remain)
end products: 2 ATP (2 lost and 4 gained), 2NADH +H, 2 Molecule pyruvate
Fate of pyruvate for animals
if oxygen is low, pyruvate is used in lactic acid fermentation
if oxygen is present, aerobic respiration continues, and pyruvate is absorbed into the mitochondrial matrix for aerobic respiration
4 stages of cellular respiration
1- glycolysis, 2-the link reaction, 3-the Krebs cycle, 4- oxidative phosphorylation
key understanding- in cellular respiration molecules are reduced and oxidized (glucose is oxidized and ADP is reduced to ATP)
aerobic respiration- requires oxygen and gives a large yield of ATP
Glucose + oxygen —-> Carbon dioxide + water + ATP energy
The Krebs cycle
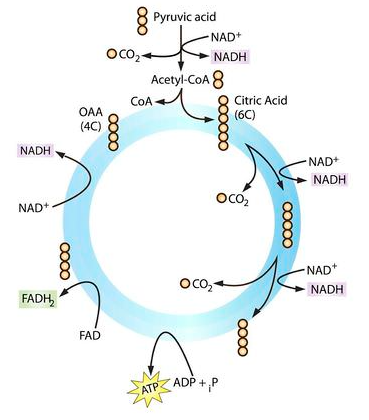
know the differences between lactic acid fermentation and alcohol fermentation
the fate of the pyruvate
if oxygen is deficient, pyruvate is used for anaerobic respiration
if oxygen is present, aerobic respiration continues, and pyruvate is absorbed into the mitochondrial matrix where it is fully oxidized
Oxidative phosphorylation
most ATP is made by ATP Synthase
this is an integral protein that’s embedded in the membrane, it aids in facilitative diffusion of h+ ions, this causes the turning of the protein which helps turn ADP to ATP
this enzyme works like a windmill, using the flow of ions to make ATP
this current that turns this windmill is generate by electron transport chain
there are many throughout the membrane and cristae
the inner membrane- holds special electron carriers to shuttle protons (ions) across the inner membrane into the Intermembrane space.
the electron carriers (proton pumps) are energized by accepting electrons from NADH and FADH2, then passing electrons down the chain
oxygen is the final acceptor in the ETC, O2 joins with the h+ ions to form water
oxidative phosphorylation is divided into the ETC and Chemiosmosis
Chemiosmosis- the action of ATP synthase, the passive transport of protons down their concentration gradients back into the mitochondrion matrix.
Mitochondria
Cristae (long pieces of the membrane that exist throughout the mitochondria)- increase surface area
Inner Membrane- is where electron transport occurs, chain reaction and chemiosmosis
outer membrane- regulates transport in and out of the mitochondria (pyruvate in and ATP out)
Inter membrane- space where H+ ions concentrate
Matrix- link reactions in the Kreb cycle occur, and mitochondrial DNA can be found here
the mitochondria contains 70s ribosomes