Chemistry - Unit 3: The Atomic Theory
* A red asterisk indicates that the topic is selectively crucial for the NYS Regents examination.
ADV. This indicates that the topic (and its subtopics, if applicable) is advanced and should be of lower priority.
Topic 3.1 - Identifying Atoms: Early Theories
The Greeks, specifically Democritus (460 B.C.E.), knew that nature and matter was composed of building blocks.
They believed that nature and matter was composed of four elements: fire, air, earth, and water.
Topic 3.2 - Identifying Atoms: Later Theories
Topic 3.2.1 - Dalton (1766–1844)
John Dalton theorized that the basic unit of matter is the atom.
Believed that all elements were composed of indivisible atoms.
Stated that all atoms of a specific element are identical. Atoms in different elements are different and have different masses.
Topic 3.2.2 - Thomson (1856–1940)
William Thomson used a cathode ray tube.
The ray produced was deflected by an electrical field.
It shows that the particle had a negative charge.
Thomson was credited with the discovery of electrons.
Topic 3.2.3 - Plum Pudding Model
A model that represented the atom as a ball of positive charge with negatively charged electrons within it.
* Topic 3.2.4 - Rutherford 1871–1937
Ernest Rutherford discovered the nucleus using the Gold Foil Experiment.
In the experiment, positively charged alpha particles were shot at a gold foil.
Some particles passed through, while others deflected and went in different directions.
Observations | Conclusions |
Most alpha particles passed through the gold foil. | Atoms mostly consist of empty space. |
Some alpha particles were deflected. | Atoms have a small, dense positive core (nucleus). |
* Topic 3.2.5 - The Bohr Model
Neils Bohr (1913) was credited with the modern atomic theory.
He developed a simplistic view of the atom.
This is also called the planetary model.
Bohr claimed that Rutherford positioned the electrons incorrectly.
He theorized that outer orbitals contained electrons.
According to Bohr, each electron must have the correct amount of energy to keep it in place around the nucleus.
Topic 3.2.6 - The Wave Mechanical Model
Electrons have energy and can act like a wave and a particle.
Electrons are found in clouds, which contain orbitals (areas that electrons are likely located based on the energy they possess).
Topic 3.3 - The Construction of the Atom
The nucleus is dense and centered.
Protons are positively (+) charged.
Neutrons are not charged at all.
Electrons are negatively charged and orbit in shells/orbitals (in the electron clouds).
No two elements have the same atom; all elements are different.
Topic 3.3.1 - The Particles of the Atom
Particle | Symbol | Charge | Approximate Mass | Location |
Proton | 11H or 11P | +1 | 1 u (amu) | Nucleus |
Neutron | 10n | 0 | 1 u (amu) | Nucleus |
Electrons | 0-1e | -1 | 1/1836 u (amu) | Orbitals |
Topic 3.3.2 - Atomic Number
The atomic number represents the number of protons in a nucleus.
It is equal to the nuclear charge.
In a neutral atom (anything on the periodic table), the number of protons and electrons are equal.
Topic 3.3.3 - Mass Number
The mass number represents the sum of the number of protons and the number of neutrons.
Round the atomic mass to obtain the mass number. The mass number is not the same as the atomic mass.
To calculate the number of neutrons, subtract the number of protons from the mass number.
Topic 3.4 - Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons and hence have different mass numbers.
They can be identified with a symbol that indicates the element and the mass number.
Topic 3.4.1 - Isotope Notations
126C: 12 is the mass number, 6 is the atomic number, and C is the element (carbon).
12C: 12 is the mass number and C is the element (carbon).
Carbon-12: Carbon is the element and 12 is the mass number.
C-12: C is the element (carbon) and 12 is the mass number.
Topic 3.5 - Ions
Ions are charged atoms. They can either be positively or negatively charged.
Positively charged atoms have more protons than electrons.
Negatively charged atoms have more electrons than protons.
The number of protons in an ion matches that of its neutral atom. Only the number of electrons changes.
Topic 3.5.1 - Cations & Anions
Cations are positively charged ions. In cations, there are fewer electrons than protons.
Anions are negatively charged ions. In anions, there are more electrons than protons.
Topic 3.6 - Atomic Masses
The atomic mass of an element is the average mass of all the isotopes of a sample of an element, measured in atomic mass units (amu or u).
Atomic masses on the periodic table are an average based on the abundance of each isotope.
The formula for calculating the atomic mass is (M1 × A1) + (M2 × A2) + …, where Mn is the mass of isotope n and An is the relative abundance (%) of isotope n.
The percent abundance is written as a decimal.
Topic 3.7 - Principal Energy Levels
Principal energy levels show how far the electron is from the nucleus.
There are four principal energy levels; each one has a different level of energy and a different electron capacity.

Topic 3.7.1 - Electron Configuration
The electron configuration of an element shows the number of electrons in each principal energy level.
Fluorine has an electron configuration of 2-7, meaning that the first energy level has two electrons, and the second energy level has seven.
When an energy level is filled, it has reached maximum capacity.
When an energy level is occupied, it contains at least one electron.
An energy level can be filled and occupied, but not vice versa.
Topic 3.7.2 - Valence Electrons
Valence electrons are electrons found in the outermost shell.
To determine the number of valence electrons, look at the last number in the electron configuration.
Topic 3.8 - Energy Levels & Orbitals
Electrons located in orbitals have the energy of that orbital.
Electrons can move to different orbitals. To move to higher orbitals, they must absorb the energy required.
Energy is absorbed in the form of heat or light.
Topic 3.8.1 - Ground State vs. Excited State
Ground state (most stable): Electrons occupy the lowest energy level possible (everything on the periodic table).
Excited state (unstable): Electrons advance to a higher energy level and leave one of the principal energy levels partly empty.
ADV. Topic 3.9 - The Aufbau Principle
The Aufbau principle is a rule used in chemistry to determine the electron configuration of atoms. It states that electrons fill atomic orbitals in a specific order, starting from the lowest energy level and moving to higher energy levels. "Aufbau" means "building up" in German, indicating that electrons build up from lower to higher energy states.

According to this principle, electrons occupy orbitals in the following order:
1s (lowest energy level)
2s
2p
3s
3p
4s (filled before 3d because it has lower energy)
3d
4p
5s
4d
5p, and so on.
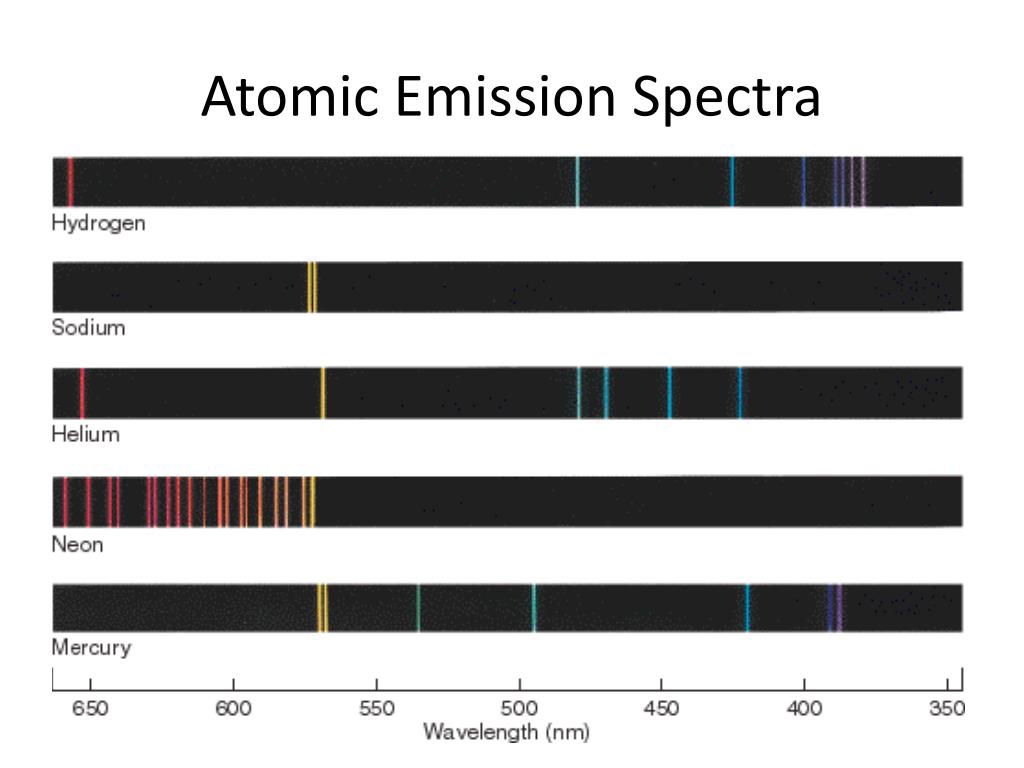
Topic 3.10 - Atomic Emission Spectra
Electrons do not stay in the excited state for a long time. They will eventually fall back down to the ground state.
The atomic emission spectra are the unique set of frequencies of electromagnetic radiation emitted by the electrons of an atom when they transition from a higher energy level to a lower energy level.
When atoms absorb energy (such as from heat or electricity), their electrons become excited and move to higher energy levels. As the electrons return to their original, lower energy levels, they release energy in the form of light. The wavelengths of this emitted light form a spectrum that is characteristic of the specific element.