lecture 3: structural basis of clathrin mediated endocytosis
most of the structures in this are crystal structures and not cryo-em
The role of clathrin and AP2
Both clathrin and AP2 are difficult proteins to work with. For clathrin, this is because of its 3 legged shape. For AP2 it is due to the number of subunits and the parts attached by the linker region.
Clathrin has an unusual three legged shape and assembles into large carge structures but is hard to crystalise. AP2 however, is composed for 4 subunits with flexible (3) domains attached by a linker region and is complex to produce to to getting all four units expressed. Both are important for clathrin mediated endocytosis and are instrumental in the formation of the cage.
clathrin mediated endocytosis starts off with AP2 binding to receptor tails from the receptor being absorbed such as an LDL2 receptor or transferrin receptor. This enables the cell to select what is bought into it. This process stimulates the formation of the vesicle formed by clathrin which is detached by dynein. The vesicle is transported and is removed by HSC70 and the coat proteins are recycled.
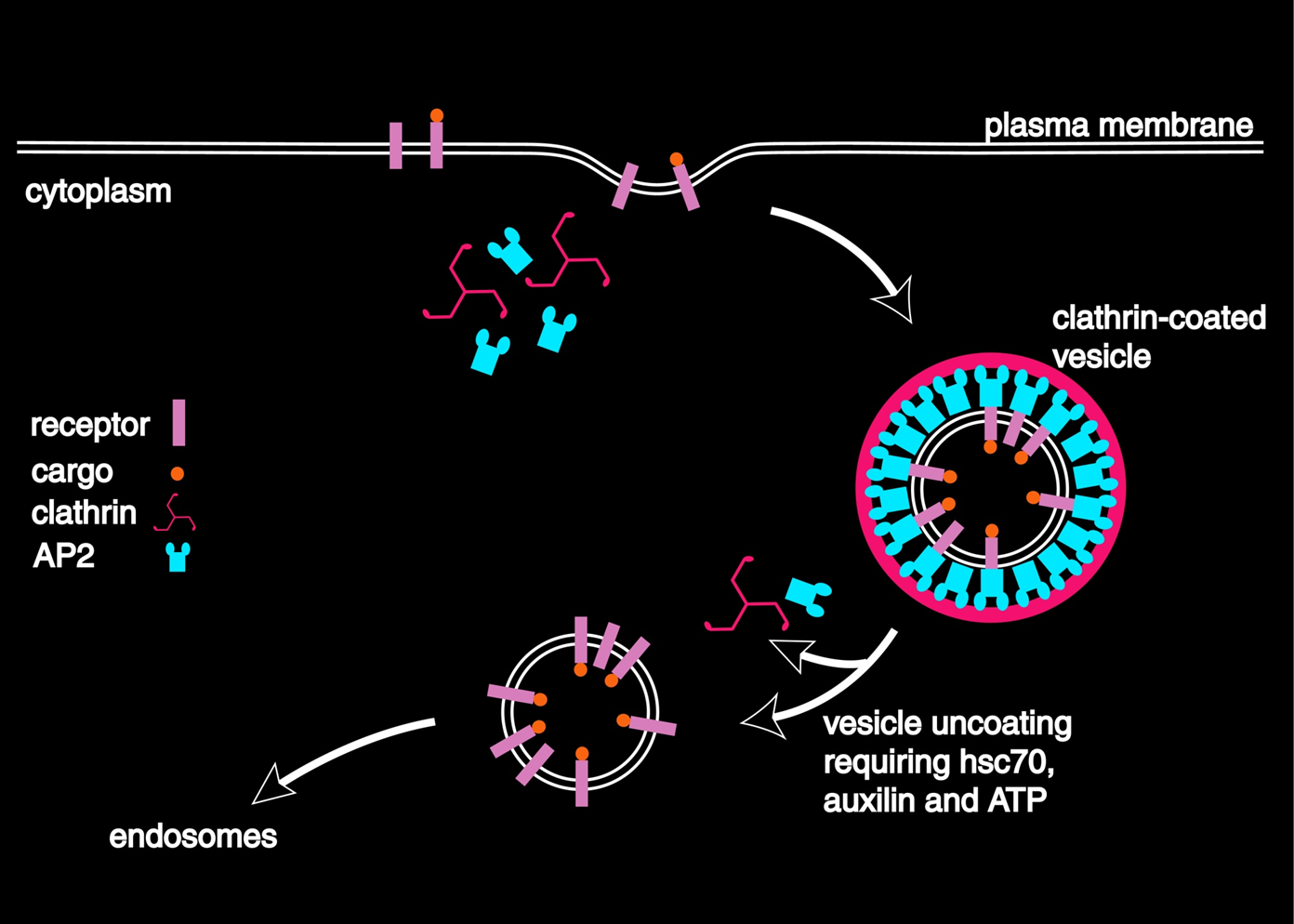
Clathrin and AP2 are at the hub of a large number of protein interactions and is important for processes such as development and in some cancers and can be purified sometimes from brain or placenta
Clathrin is composed out of a terminal domain which is important for making binding interactions and a light chain both of which are joined by a trimerisation domain in the middle. There is a light chain that comes out of the trimerisation domain. It has a characteristic 3 legged structure and can spontaneously assemble into cages when purified and put into a buffer that contains Mg2+ or Ca2+ at a pH of 6-6.5. The terminal domain is important for binding interactions and does this by a specific motif called the clathrin binding motif that is found in structures that bind to it. They are made up of 2 hydrophobic amino acid residues, a polar amino acid residue, another amino acid that doesn’t have to be specific and finally a polar one. This is called the clathrin box and examples of some verified clathrin box motifs are in beta arrestin 1 which has the domain IEFD and AP1 beta 1 subunit LLNLD.
structure of the terminal domain
The terminal domain has 2 distinct domains.
The first is a beta propeller domain that has made up of beta sheet that forms that 7 domains/blades. This is the primary site for adaptor interactions. It binds the clathrin box motif of b2 adaptin hinge region of AP2. The second is a linker domain which is consists of alpha helices that form a zig zag like structure of different lengths. The individual beta sheets form the beta propeller and peptides can bind themselves between two blades in an extended conformation. The beta propeller domain can also have other binding sites which is discussed later. Knockout experiments revealed 4 sites.
Not all sites had the same kind of interaction. The W box site is different to the beta propeller interaction and instead binds a W box that has two tryptophans at the top of the beta propeller
The 4th site was discovered and is called the royle box. this revealed that some clathrin binding peptides used in these experiments bound to multiple sites shown that proteins can bind to multiple sites. One clathrin binding domain can bind to AP2. The final binding site is called the arrestine site
cryo-em of clathrin cages
The process of getting a structure was long and started in 1969 with a negative stain and the final structure was discovered in 2004 with the highest resolution.
This was done with cryo-em by purifying it and putting it in a buffer with the correct conditions to produce the cages. This is helpful as you know there is only one protein. with adaptor proteins, further formation are promoted. the cages formed are heterogenous and so the actual structure of the cage is random and may not be symmetrical. A cryo-em of 7.9 angstroms allowed a model of the clathrin structure to be made showing location of the light chains and a helical tripod was revealed which is a highly sable coiled coil. It has to hold the three legs together and is tight interaction. Other structures were also solved by exploiting the heterogeneity in the solution. This also allowed tracking of how different cages formed
what was observed was through making a contact map that has the amino acid residues of a leg on the y axis and on the x is the same for another leg. It was plotted where the legs interacted. This reveled that the legs were giving the same patterns of contact in all the legs. This means that legs flex to adapt to different environments but keep contacts the same. The flexibility is what allowed the different structures to form.
AP2 domain interactions
AP2 scanning em images revealed that it has a core and apendange domains which were names “ear” domains. It is a hetero tetramer composed of 4 domains. The a and b domains are the biggest and have the ear domains attached by a linker region. there is also a sigma domain and a mew domain which is responsible for the interaction of it with its receptor tail. AP2 is capable to multiple interactions including clathrin which has an interaction site on the b2 domain and a clathrin box on the hinge domain
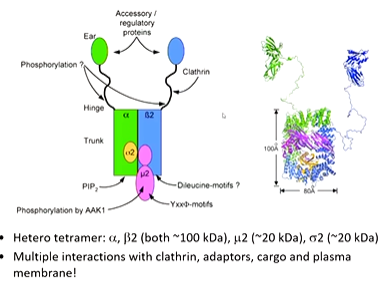
Crystallography did not work initially so the protein was broken up to analyses. The a and b2 ear domains are similar in structure it have different binding interactions. They are multiple binding sites for short protein motifs that bind in an extended way ut there is one alpha helix that can bind.
The x ray structure of the mew 2 domain is important as it is responsible for beginning the right thing into the cell. It is a stable domain and solves the structure with peptides that were complementary to the receptor tails. The consensus sequence was then called the YXXphi sequence (Y amino acid, another 2 random ones and then a hydrophobic amino acid)
Specificity is achieved through a linear peptide motif and the peptide bound (YXXphi motif). The surface of this domain has a strong electrostatic potential which encourages binding to membranes which are negatively charged.
AP2 core structure and function
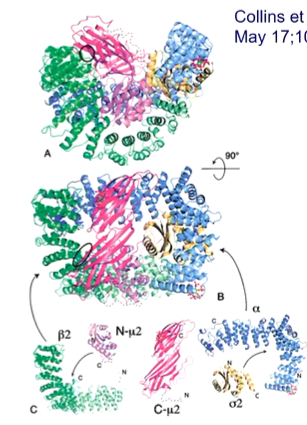
The atomic structure of AP2 trunk took years to achieve as specific protein expression was required that assembled properly. It is composed of 2 curved super-helical solenoids - a and b2 trunk domains. and each forms an elbow containing sigma 2 and N-meu 2 domains. The C - meu 2 domain rests on a shallow dish formed by all the 4 resting domains.
The only problem was that the crystal structure was not functional and could not bind receptor tail binding motifs. This lead to a model for closed and open states of AP2. AP2 is known to bind to PIP3 and PIP2. It was crystallized in the presence of a phospholipid analouge - IP6 - to achieve crystallization. This enabled to visualize where IP6 was binding to see how AP2 changes to bind to the membrane. The model suggests that the open structure has the meu 2 domain flips open to reveal the YXXphi binding site.
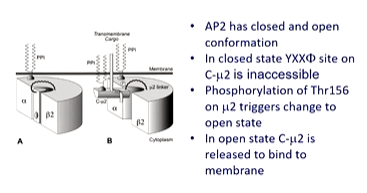
A further crystal structure was done with another binding site and reveled that a different binding motif - dileucine - could also bind on the sigma subunit and this has a different motif. This has a different conformation of AP2 on its way to opening out fully. On the fully open state, the dileucine and YXXphi are fully open to capture receptors.
In further papers, they realised that clathrin binding motif on the hinge region on the b2 subunit was hidden in some subunits and was exposed when the complex could bind cargo and the membrane. This means that this triggers a conformational change that binds clathrin. ‘
cryo-em confirmed all of this.