Topic 1 - atomic structure and the periodic table
The atom is made up of three types of sub-atomic particles: Protons, neutrons and electrons. These particles help decided the chemical properties and behaviour of the atom.
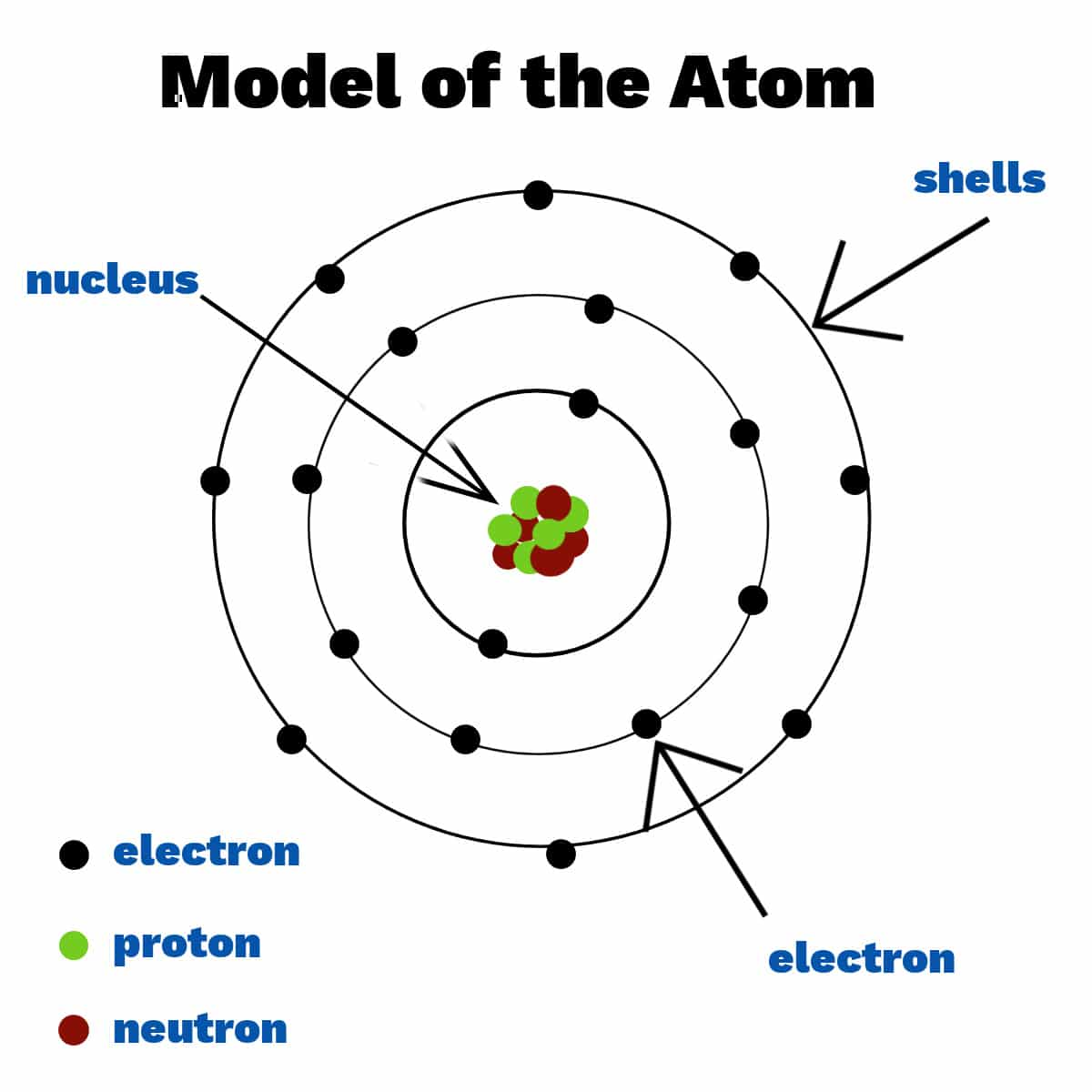
The mass number is the number of protons and neutrons added together, as they both have a mass of one. The atomic number is also known as the proton number as it tells you how many protons there are in the atom (thereby also the number of electrons)
The modern periodic table is sorted by atomic number
How does mass spectra work
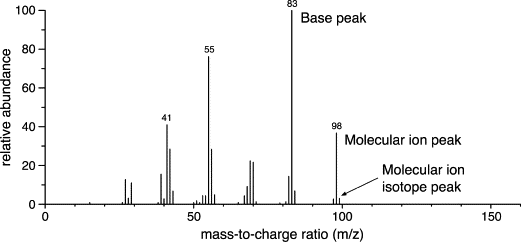
The X-Axis shows the m/z value which is the mass to charge ratio
Y-Axis shows the relative abundance of each ion
For molecular samples the m/z at the highest peak is the ion
The m/z of the molecular ion is the relative molecular mass
How to calculate Ar (relative atomic mass)
Multiply the relative isotopic mass by the relative abundance for each isotope
Add the answers
Divide by 100 (to find a percentage)
An element us a substance made from one type of atoms, an isotope of that element has the same number of protons but a different number of neutrons. Because isotopes have the same electron configuration they therefore have the same chemical properties.
Relative isotopic mass → Mass of an atom of an isotope compared to 1/12th of a carbon-12 atom.
Relative atomic mass → Average mass of an atom of an element compared to 1/12th of a carbon-12 atom
First ionisation energy
First ionisation energy → amount of energy required to remove an electron from each atom in one mole of gaseous atoms (to form one mole of gaseous 1+ ions)
Ionisation energy is affected by:
Nuclear charge - having more protons will mean there is a stronger positive charge so there is stronger electrostatic attraction
Atomic radius - The larger the atomic radius, the weaker the electrostatic attraction
Shielding - Outer electrons are more shielded so they experience less
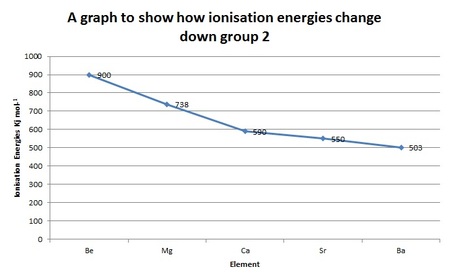
In group 2 the nuclear charge increases down the group
The atomic radius increases down the group
Shielding increases down the group
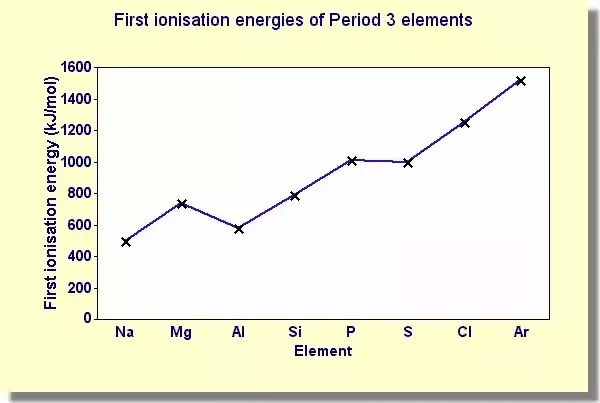
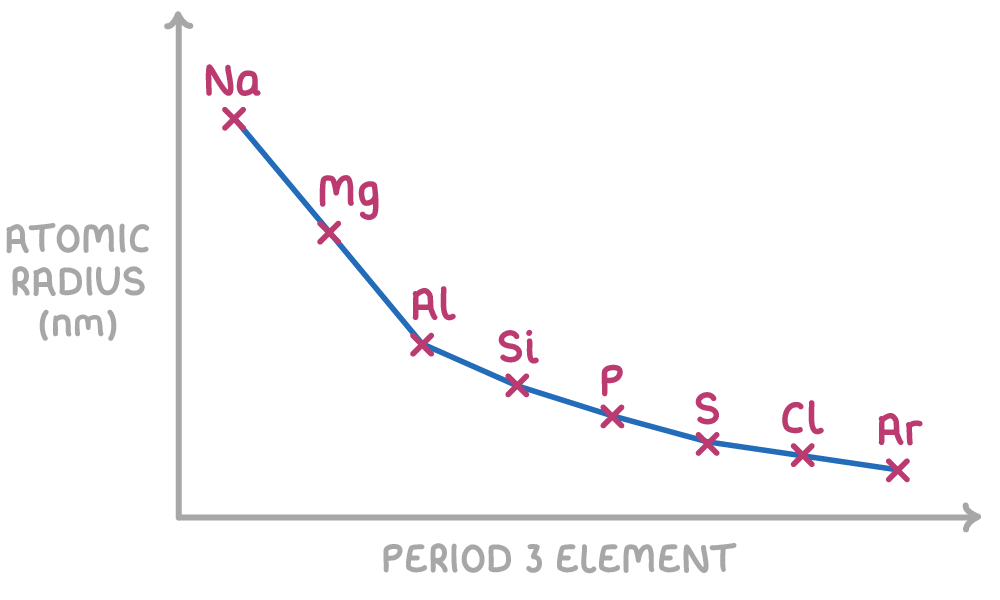
Across a period the first ionisation energy generally increases as nuclear charge increases but atomic radius decreases and shielding doesn’t change to have a significant effect however there are some unexpected results
Al - Aluminium is lower that Mg because the first electron is being taken from the 3p orbital as opposed to the 3s orbital like Na and Mg, the 3p orbital is slightly further away from the nucleus than the 3s orbital and therefore less energy is required to remove the first electron
S - Silicon is lower than K because of electron pairing. Both of the electrons are being taken from the 3p orbital however with silicon the electrons have started pairing up so there are two on one of the spins. These electrons are repelling each other as they both have a negative charge making it easier to remove the electron from silicon.
Successive ionisation energies
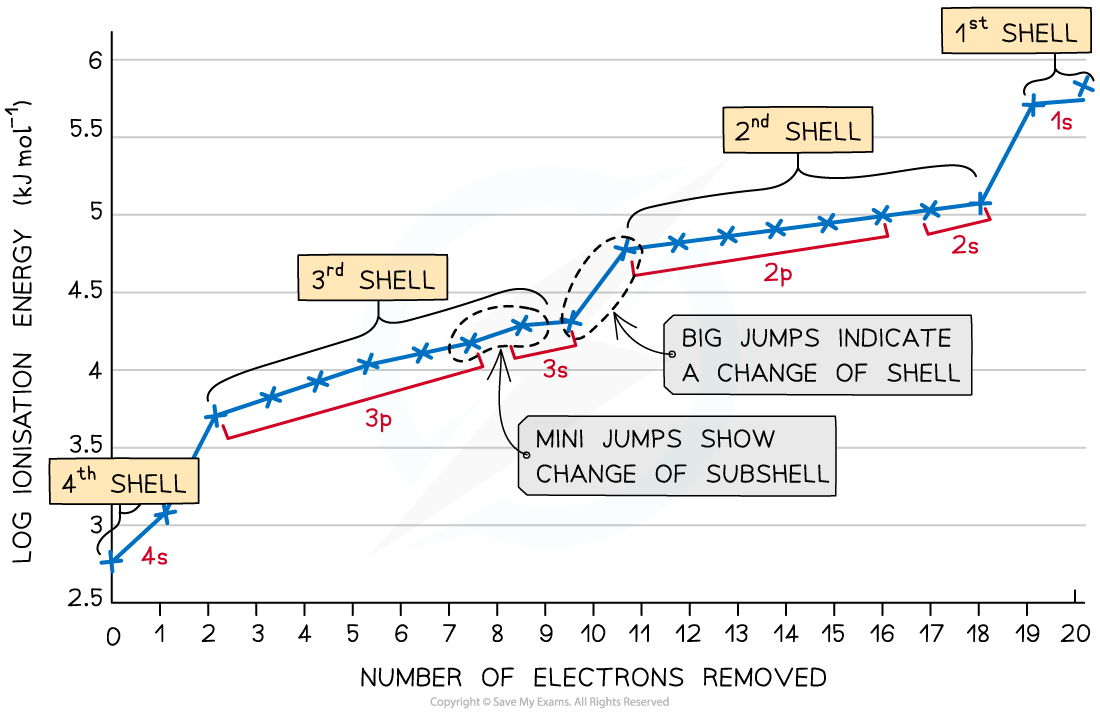
As more electrons are removed there is greater electrostatic attraction for the remaining electrons. As it moves to each new shell there is more attraction
S-orbitals are spherical but p-orbitals are shaped like an 8
Chromium and copper both have unusual electron configurations as they both use the 4s orbital before filling the 3d orbital.
Melting and Boiling points
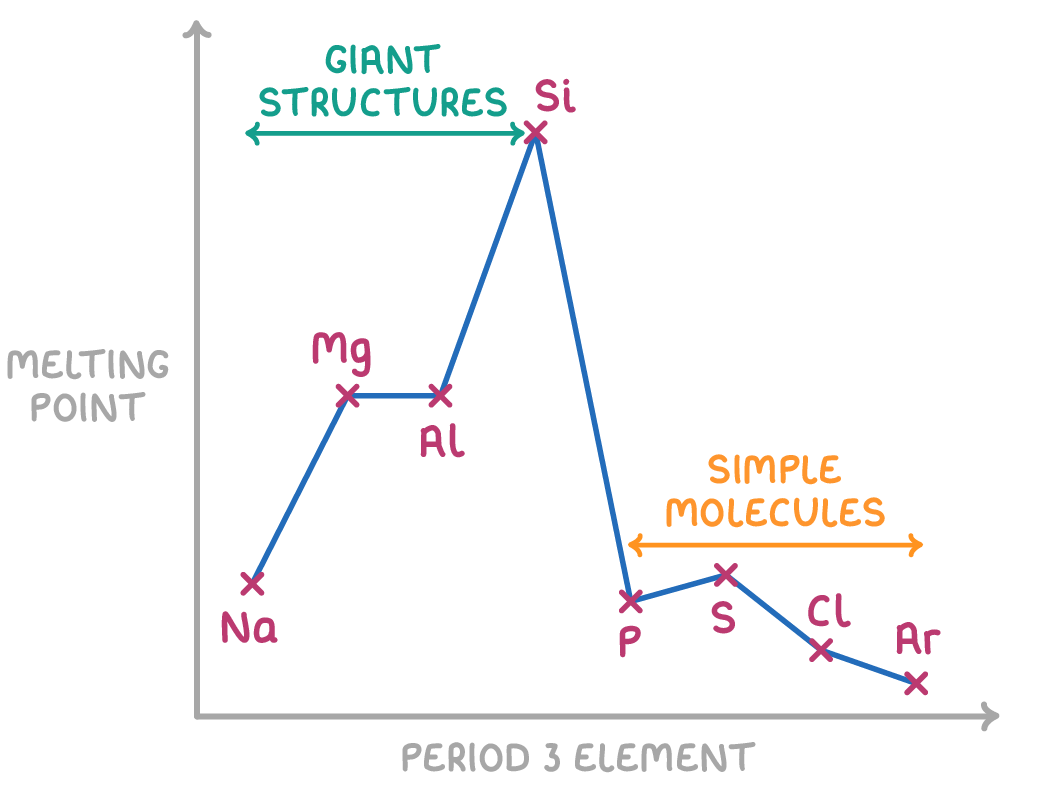
Giant structures -
Giant structures consist of elements with metallic bonding and elements with giant covalent lattice structures.
Metallic bonding increases strength across a period because the ions have a larger positive charge, smaller ionic radius, more delocalised electrons and thereby stronger electrostatic attraction
Giant covalent structures have very veryyyyy strong covalent bonds holding them together
Simple molecules -
Simple molecules are not held together by strong bonds but rather weak intermolecular forces (induced dipole-dipole) between the molecules.
Nobel gases have no bonds and weak induced dipole-dipole forces.
The atomic radius decreases across a group because nuclear charge increases as protons are added, there is no shielding for inner electrons, stronger electrostatic means the atomic radius decreases