Metals Reactions
1. Reactivity Series of Metals
Reactivity Order: Potassium > Sodium > Calcium > Magnesium > Aluminium > Carbon > Zinc > Iron > Hydrogen > Copper > Silver > Gold
Key Trends:
The reactivity decreases down the series.
Highly reactive metals react vigorously, while less reactive metals show little or no reaction under similar conditions.
2. Reactions of Metals with Water
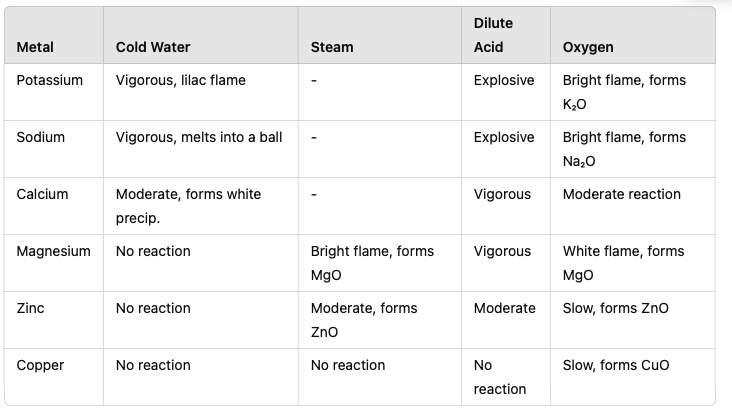
Cold Water:
Metals like potassium (K), sodium (Na), and calcium (Ca) react vigorously with cold water.
Products: Metal hydroxide and hydrogen gas.
Examples:
Potassium:2K+2H2O→2KOH+H2 Observation: Potassium ignites with a lilac flame, forming bubbles of hydrogen gas.
Calcium:Ca+2H2O→Ca(OH)2+H2 Observation: Calcium reacts less vigorously, forming a cloudy solution (due to insoluble Ca(OH)₂).
Steam:
Metals like magnesium react with steam to form metal oxides and hydrogen gas.
Example:Mg+H2O (g)→MgO+H2(g) Observation: Bright white flame and white solid (magnesium oxide) forms.
No Reaction with Water:
Metals lower in the reactivity series, such as zinc, iron, and copper, do not react with water under normal conditions.
3. Reactions of Metals with Acids
Metals react with dilute acids (e.g., HCl, H₂SO₄) to produce a salt and hydrogen gas.
Equation:Metal+Acid→Salt+H2
Examples:
Magnesium and hydrochloric acid:Mg+2HCl→MgCl2+H2
Observation: Bubbles of hydrogen form rapidly; the reaction is exothermic.
Zinc and sulfuric acid:Zn+H2SO4→ZnSO4+H2Observation: Effervescence, moderate reaction speed.
Copper and hydrochloric acid:
No reaction occurs because copper is below hydrogen in the reactivity series.
Testing for Hydrogen:
Collect the gas in a test tube and ignite with a lit splint. A ‘pop’ sound confirms the presence of hydrogen.
4. Reactions of Metals with Oxygen
Metals react with oxygen to form metal oxides. The reactivity decreases down the series.
Highly Reactive Metals:
Potassium:4K+O2→2K2O Observation: Vigorous reaction with a bright flame.
Moderately Reactive Metals:
Magnesium:2Mg+O2→2MgO Observation: Intense white flame producing a white powder.
Unreactive Metals:
Metals like copper react slowly, requiring heat:2Cu+O2→2CuO2Cu+O2→2CuO Observation: Black copper(II) oxide forms.
5. Displacement Reactions
A more reactive metal can displace a less reactive metal from its compound.
Example:
Zinc displacing copper from copper(II) sulfate:Zn+CuSO4→ZnSO4+Cu Observation: Copper metal (reddish-brown) deposits, and the solution turns colorless.
6. Practical Observations and Trends
Tendency to Form Ions:
Metals at the top of the reactivity series lose electrons more readily, forming positive ions quickly.
Barriers to Reactivity:
Aluminium appears unreactive due to its oxide layer, which prevents further reaction:4Al+3O2→2Al2O34Al+3O2→2Al2O3
Experimental Tests:
Reaction Speed: The speed of reaction with acids or water increases with reactivity.
Hydrogen Test: Confirm hydrogen gas with the “pop” test.
Summary Table