Introduction to Modern Physics
History of Atomic Models
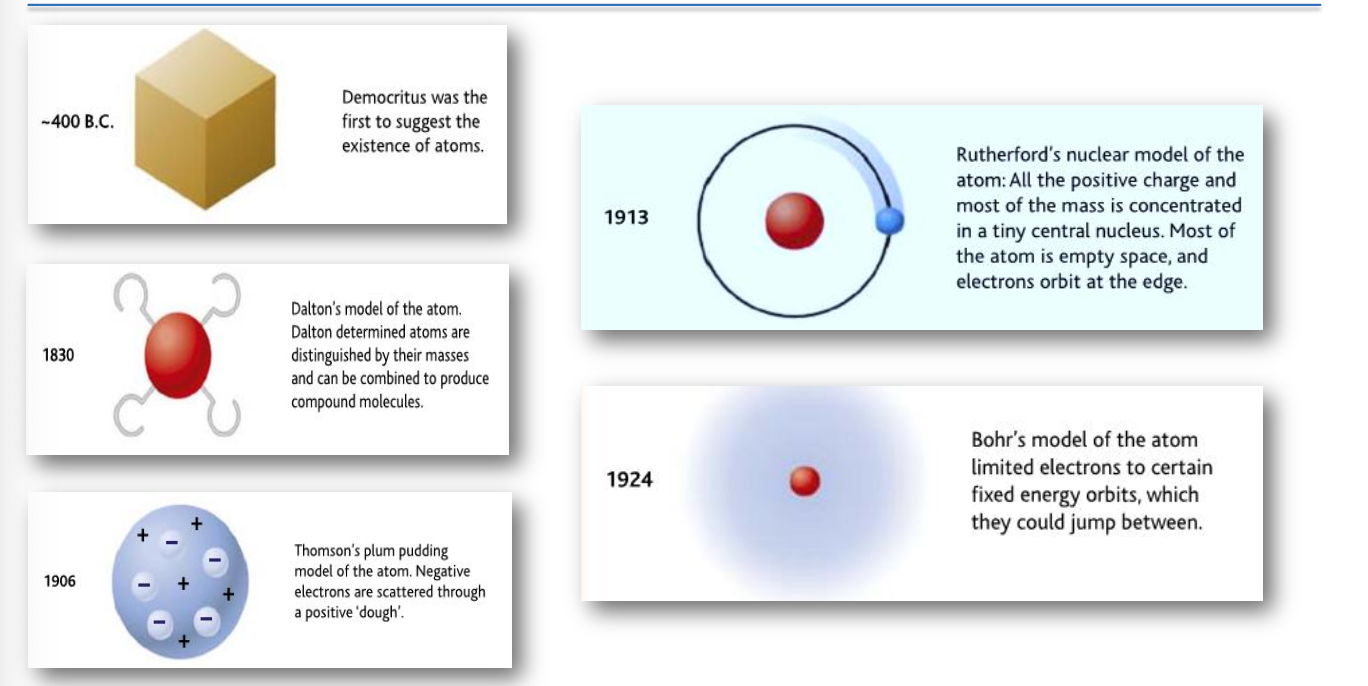
400 B.C. - Democritus
Democritus was the first philosopher to propose that matter is composed of tiny, indivisible particles known as atoms. He theorized that atoms vary in size, shape, and mass, and that they move through the void of space. His ideas laid the groundwork for atomic theory, emphasizing that these particles are the building blocks of all material substances.
1830 - Dalton's Model
John Dalton developed a systematic atomic theory, leading to the formulation of several postulates. He posited that elements are made of atoms, all of which of a given element are identical in mass and properties. Dalton also claimed that atoms can combine in simple whole-number ratios to form compounds. This marked a significant step in chemistry by establishing the idea of chemical reactions as rearrangements of atoms.
1906 - Thomson's Plum Pudding Model
J.J. Thomson proposed that the atom is a uniform sphere of positively charged 'dough' with negatively charged electrons embedded within it, similar to plums in a pudding. This model suggested that the positive charge outweighed the negative charges dispersed throughout the atom. Thomson's model was notable for introducing the concept of subatomic particles, fundamentally changing how atoms were understood.
1913 - Rutherford's Nuclear Model
Ernest Rutherford conducted the gold foil experiment, a pivotal moment in atomic theory. He found that a small fraction of alpha particles were deflected at large angles, leading to the conclusion that atoms consist of a dense nucleus made up of protons, with electrons orbiting at varying distances. This discovery challenged the earlier models that depicted the atom as a solid sphere and introduced the concept of a nuclear structure with mostly empty space surrounding the nucleus.
1924 - Bohr's Model
Niels Bohr advanced atomic theory by introducing the concept of quantized energy levels for electrons. In his model, electrons occupy specific orbits around the nucleus and can jump between these orbits when they absorb or emit energy. This model explained the stability of atoms and provided a theoretical framework for understanding atomic emission and absorption spectra.
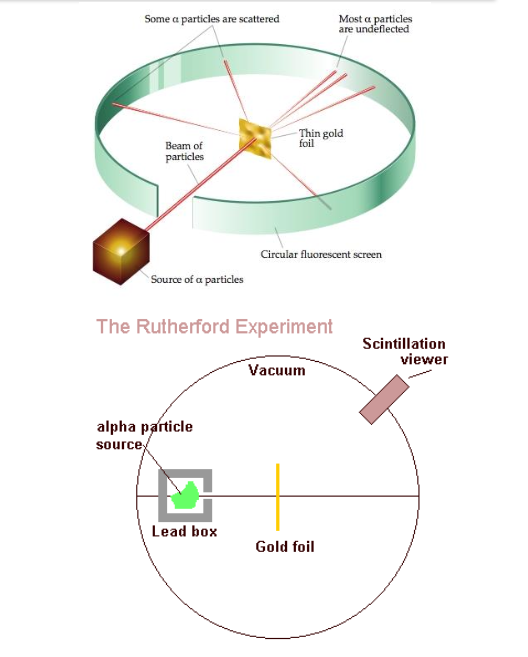
Rutherford's Gold Foil Experiment
Experimental Setup
In his groundbreaking experiment, Rutherford directed alpha particles at an ultra-thin foil of gold, known for its malleability and thinness, to enable scattering experiments. These alpha particles passed through the foil with a detection method employing a fluorescent screen that briefly illuminated when struck by alpha particles, allowing researchers to observe their trajectories.
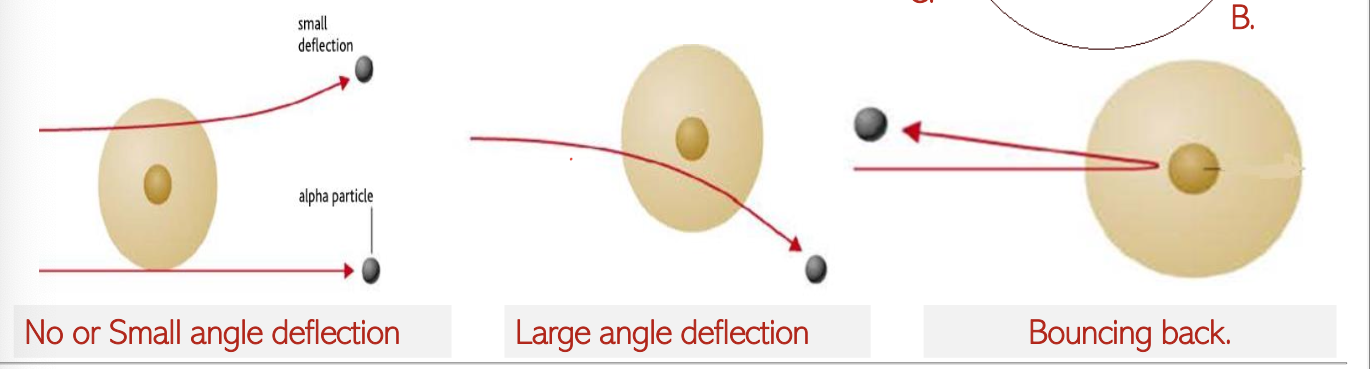
Key Observations
Result 1: Most alpha particles passed through the gold foil undeviated.
Conclusion: Most of the atom is composed of empty space, revealing that atoms are predominantly void rather than filled with mass.
Result 2: Some of alpha particles were deflected from their path (0 to 90o) but continued through the gold foil.
Conclusion: All the positive charge inside atom is concentrated and not uniformly distributed.
Result 3: A minute fraction of alpha particles (1/10,000) were bounced back.
Conclusion: Most of the mass, and all positive charge is in a tiny central nucleus.
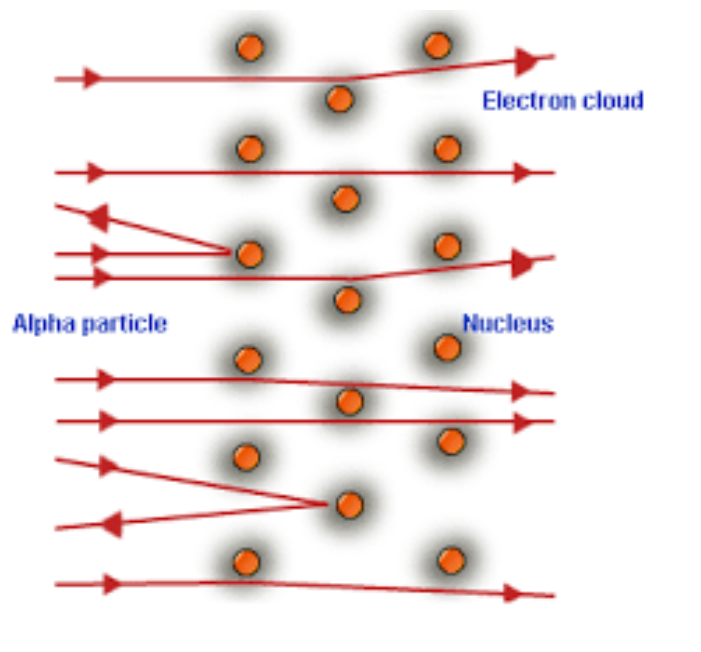
Atomic Structure According to Ernest Rutherford
Nucleus: Tiny, massive core responsible for the vast majority of the atom's mass and contains positively charged protons and uncharged neutrons—has a positive charge.
Electrons: Negatively charged particles that orbit around the nucleus, their energy levels defined by specific orbits.
Volume: The empty space between the nucleus and the electrons takes up most of the volume of the atom.
Protons and Mass Calculations
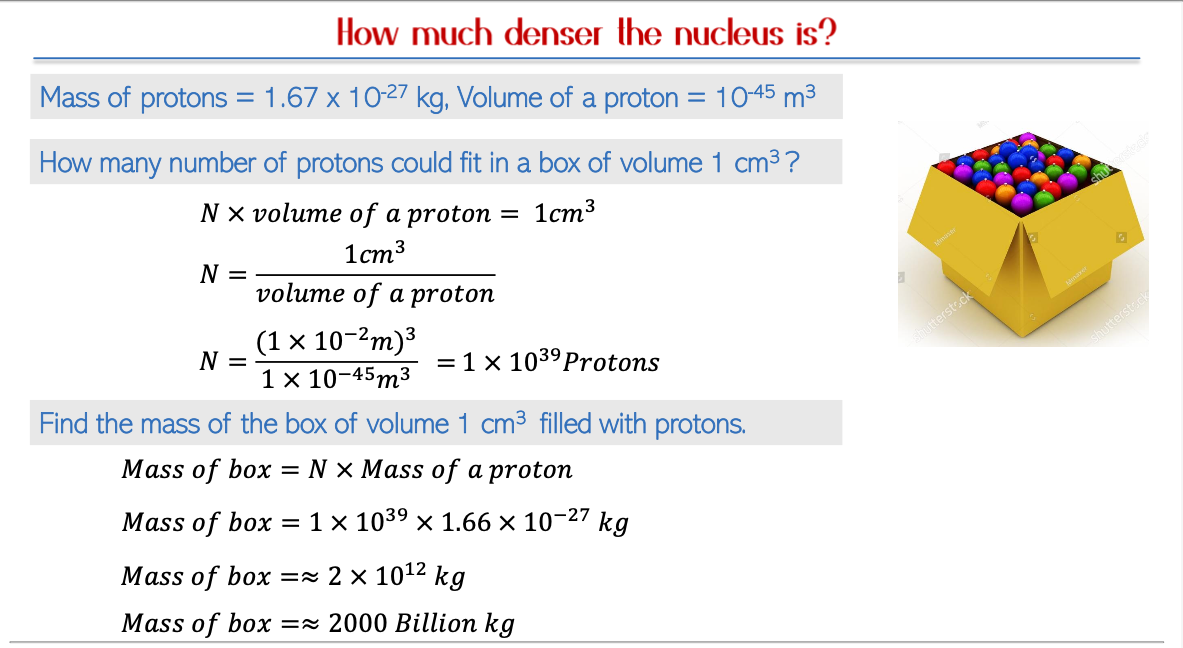
Atomic Composition
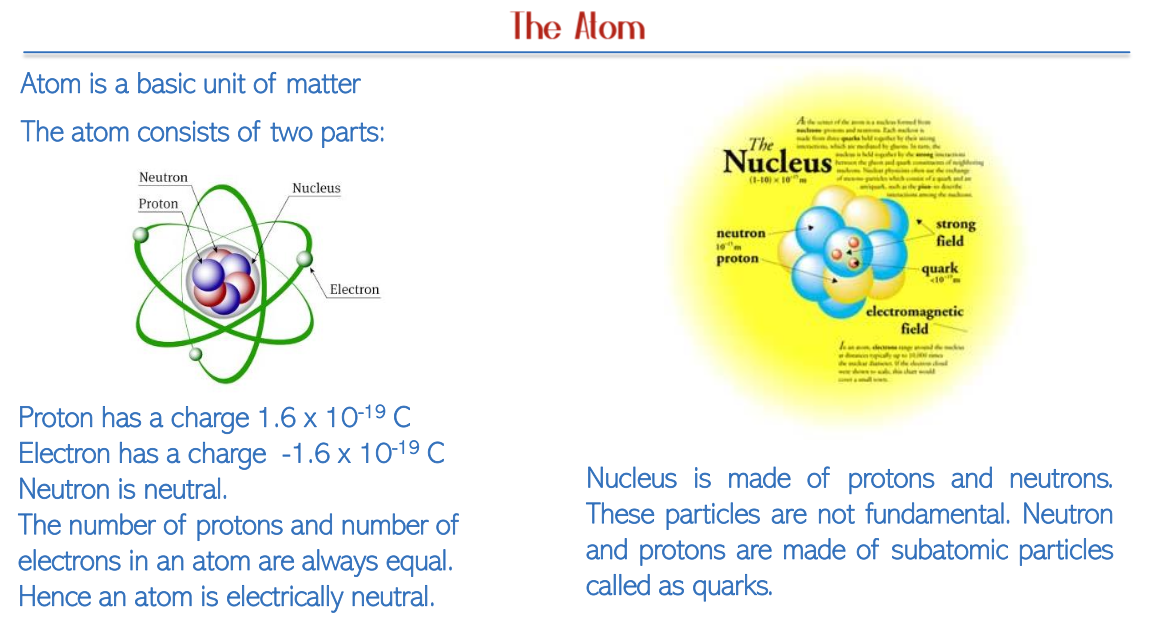
Parts of an Atom:
Protons: Positively charged particles characterized by a charge of +1.6 x 10^-19 C, contributing to the atomic number and thus determining an element's identity.
Electrons: Negatively charged particles that orbit the nucleus, each with a charge of -1.6 x 10^-19 C, balancing the positive charge of the protons.
Neutrons: Subatomic particles with no charge that play a crucial role in contributing to the mass of the nucleus without affecting the net charge.
Atoms achieve electrical neutrality due to an equal number of protons and electrons, highlighting the balance of forces within atomic structure.
Nucleus Composition: The nucleus houses both protons and neutrons. Importantly, these are not fundamental particles; rather, they are made up of even smaller constituents called quarks, which are held together by the strong nuclear force.
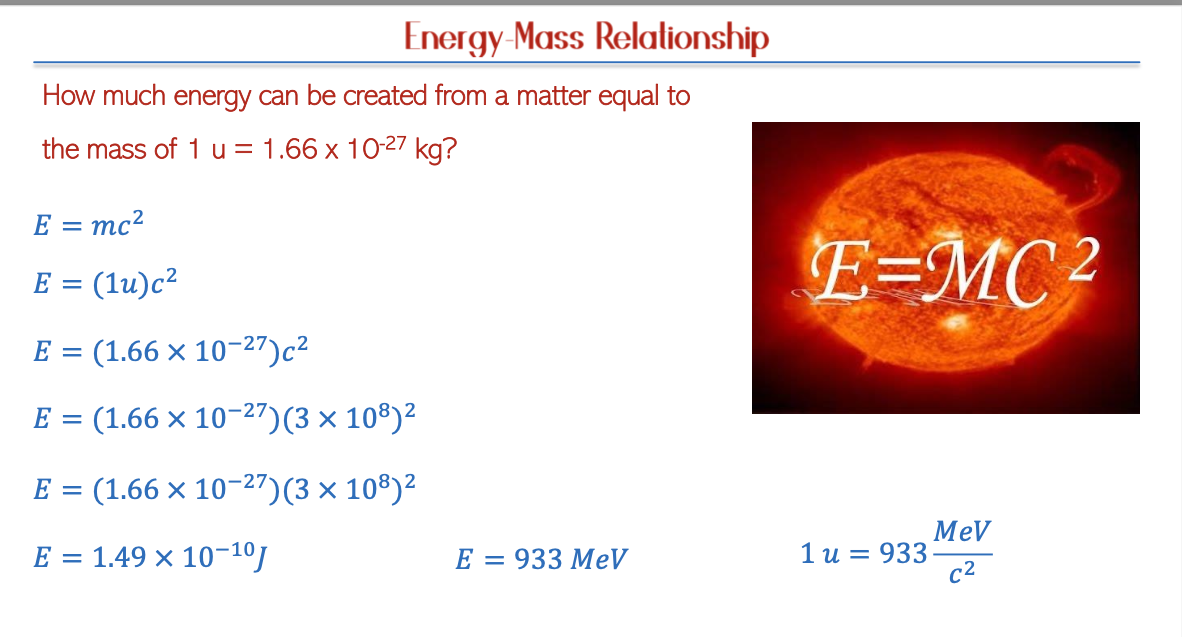
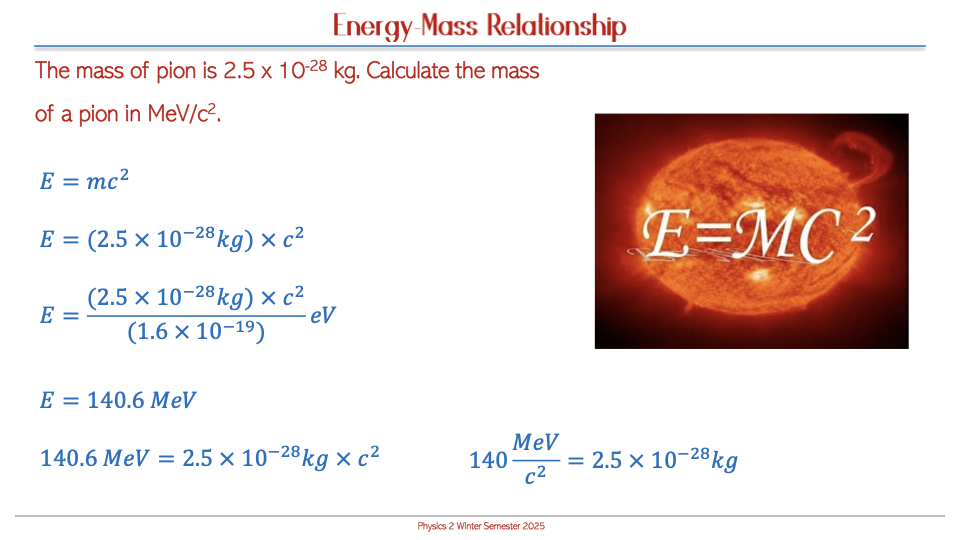
Energy-Matter Interconversion
Einstein’s Equation
According to Einstein's theory of relativity, matter can be converted into energy, as represented by the equation E = mc².
“When a mass travels with speed of light, it becomes energy”
This is WRONG

Quarks and Particle Physics
The Standard Model
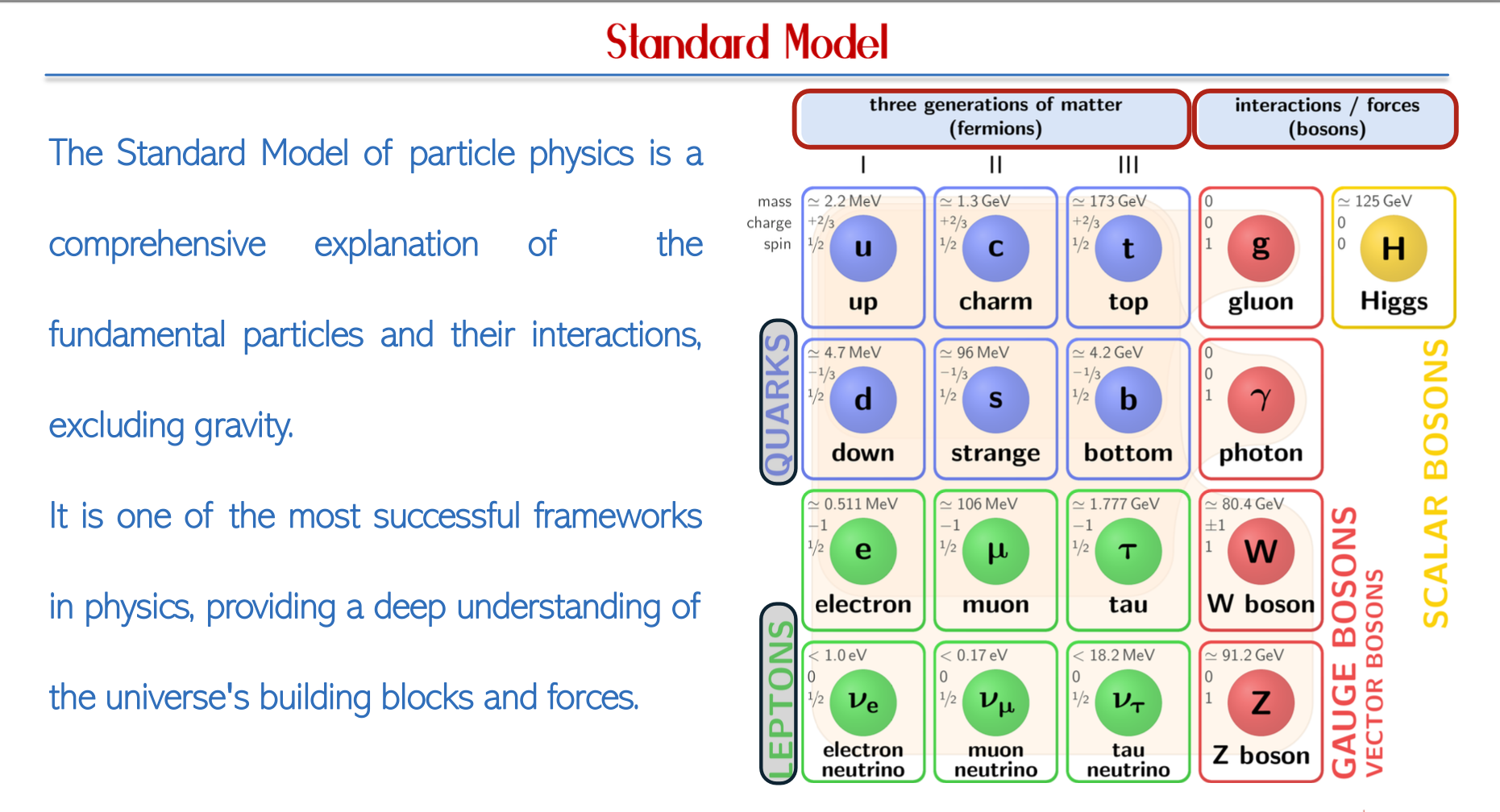
Fundamental Particles (Fermions)
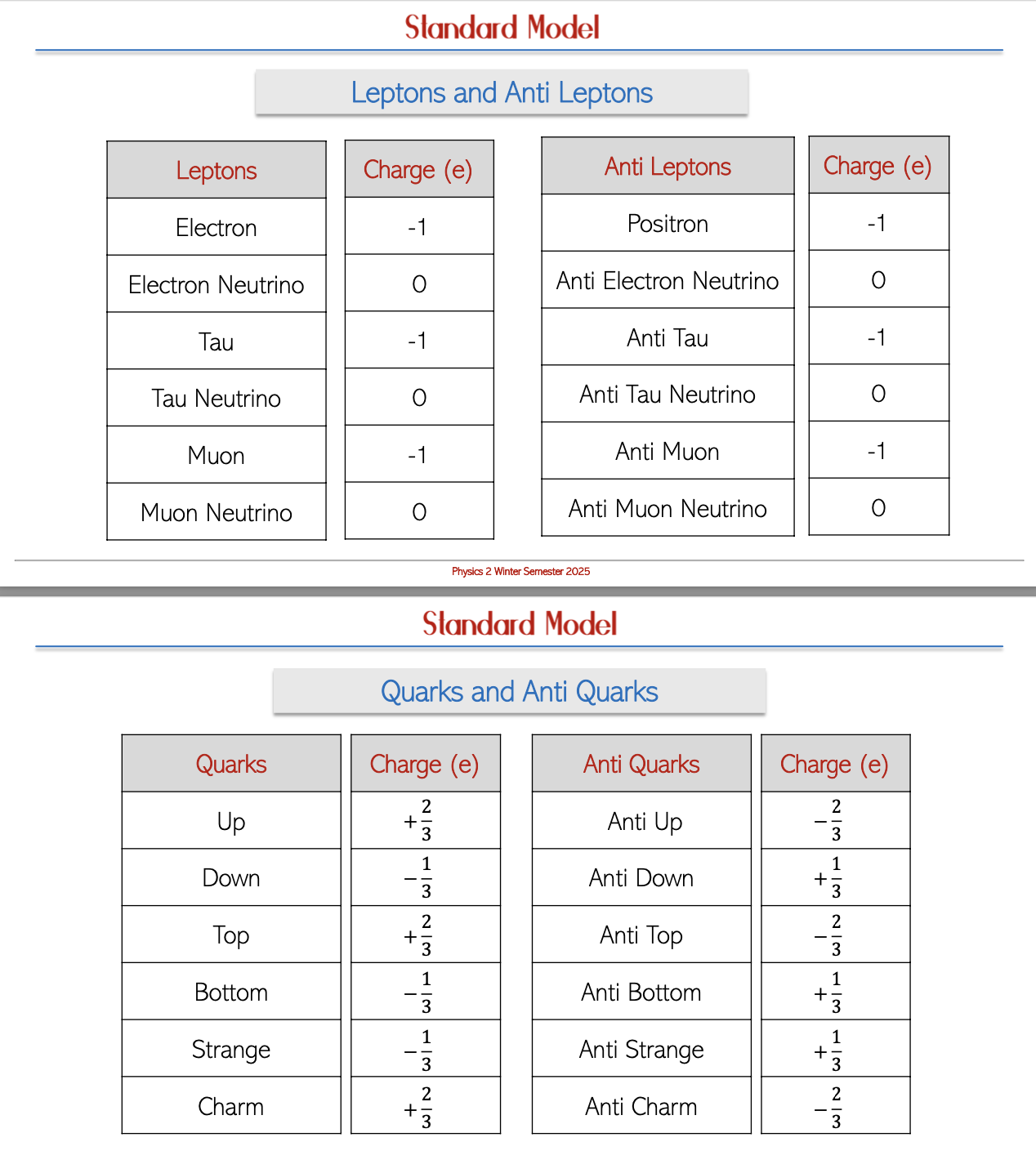
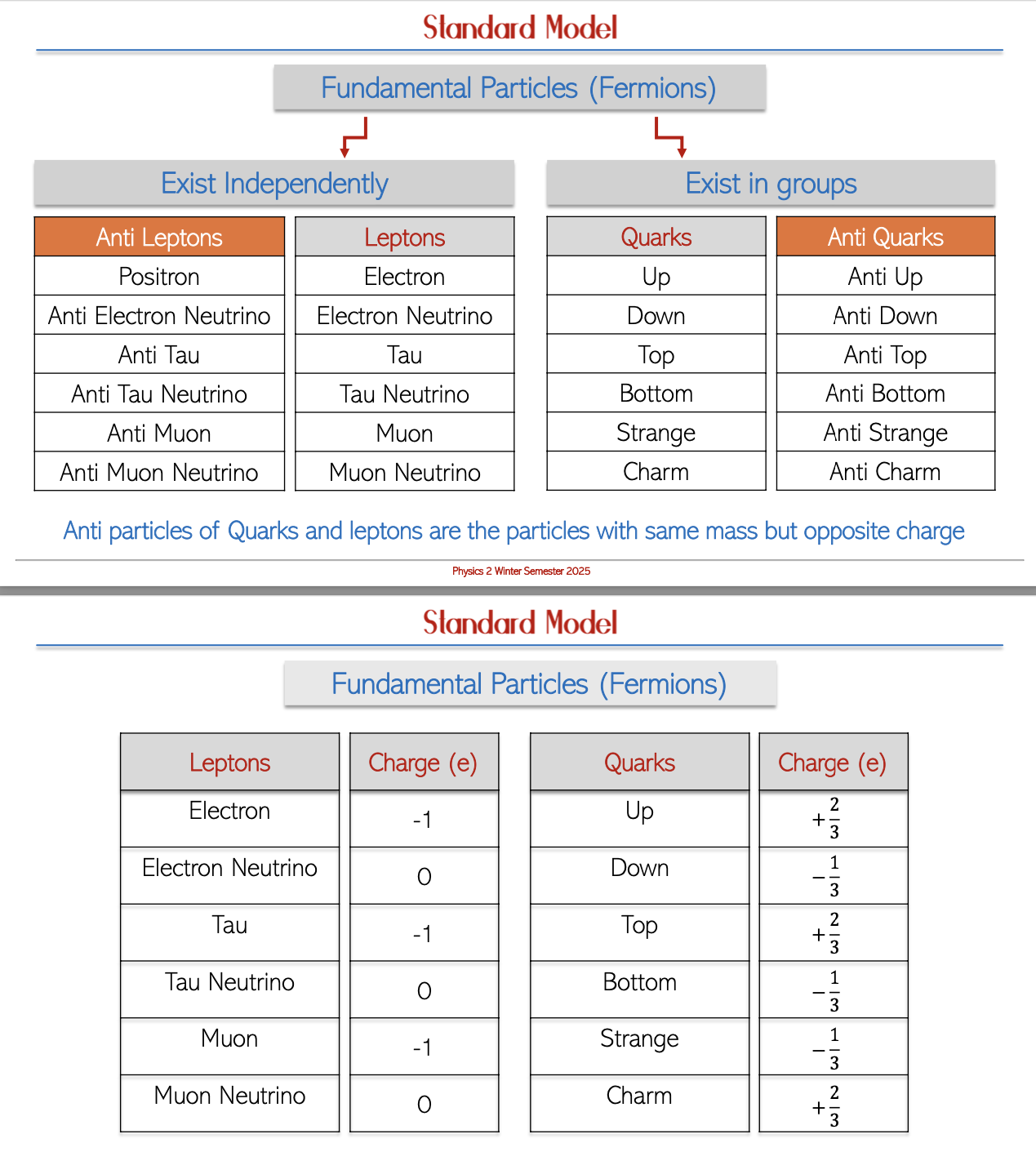
Leptons: This category encompasses electrons, electron neutrinos, tau particles, and their antiparticles, which are critical in particle interactions.
Quarks: There are six types of quarks that combine to form more complex particles:
Up (u)
Down (d)
Strange (s)
Charm (c)
Top (t)
Bottom (b)
Anti-Particles: Each lepton and quark has an oppositely charged counterpart known as an anti-particle, fundamental to the principles of particle physics and conservation laws.
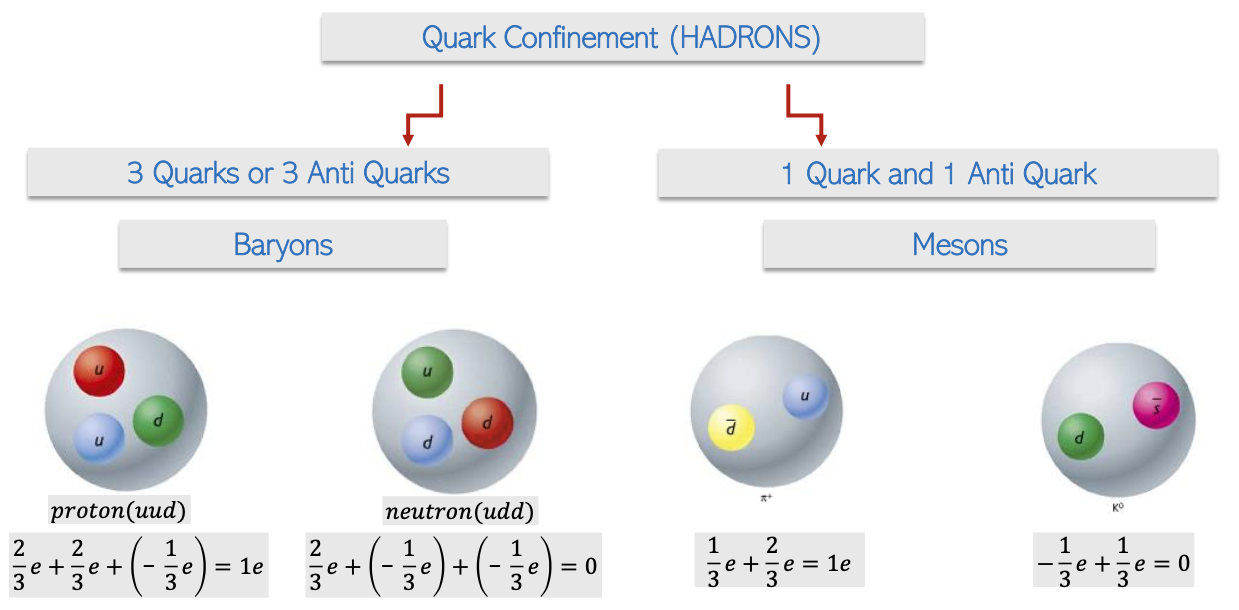
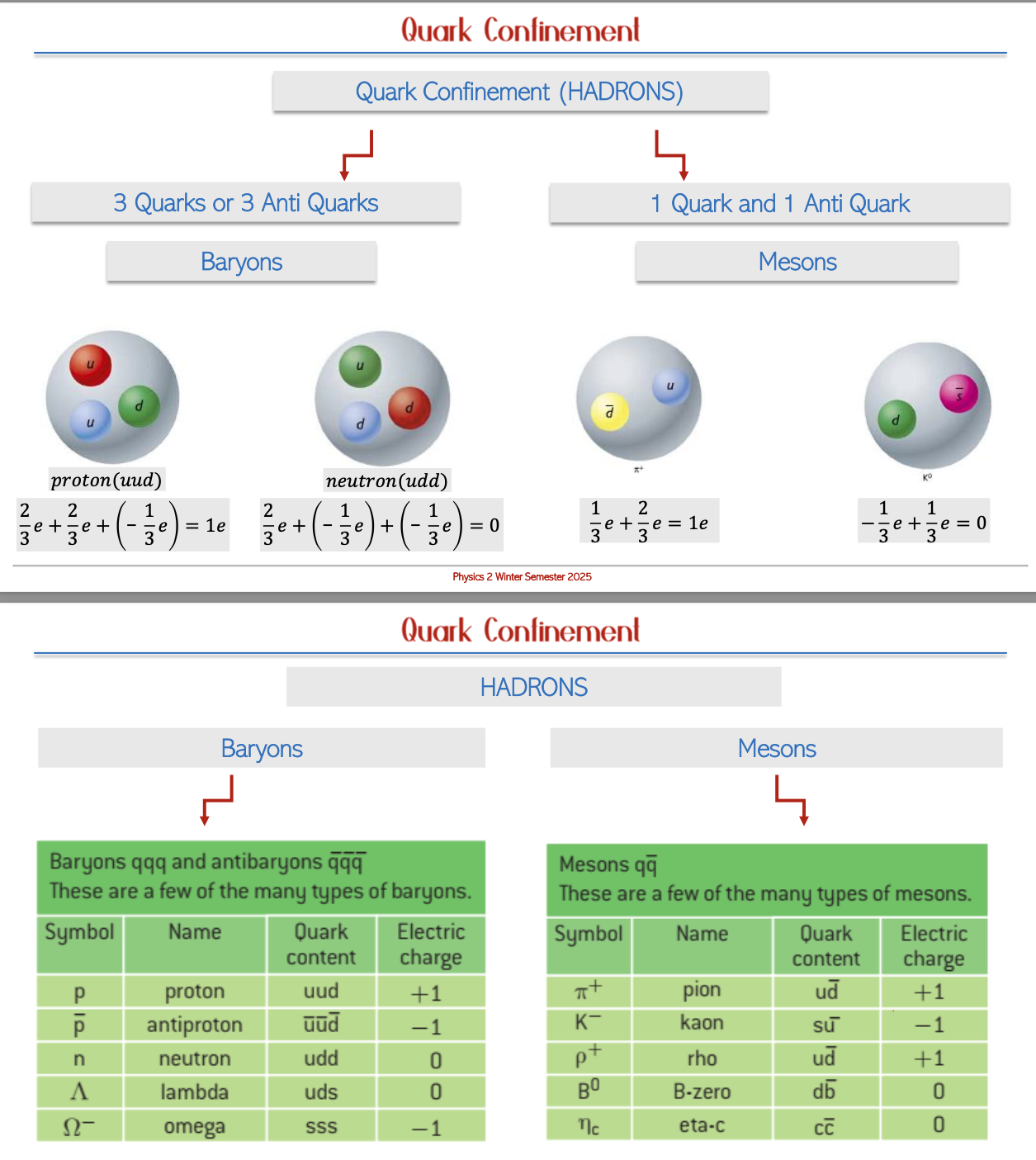
Quark Combinations and Confinement
Baryons: These composite particles consist of three quarks, including the protons and neutrons that make up atomic nuclei, showcasing the intricate structure of matter.
Mesons: Formed from a combination of one quark and one anti-quark, mesons play a role in mediating the strong force within atomic nuclei.
Quark Confinement: Quarks are never found in isolation; they are inherently bound within larger particles—either baryons or mesons due to strong force interactions, illustrating the complexities of particle interactions in nature.


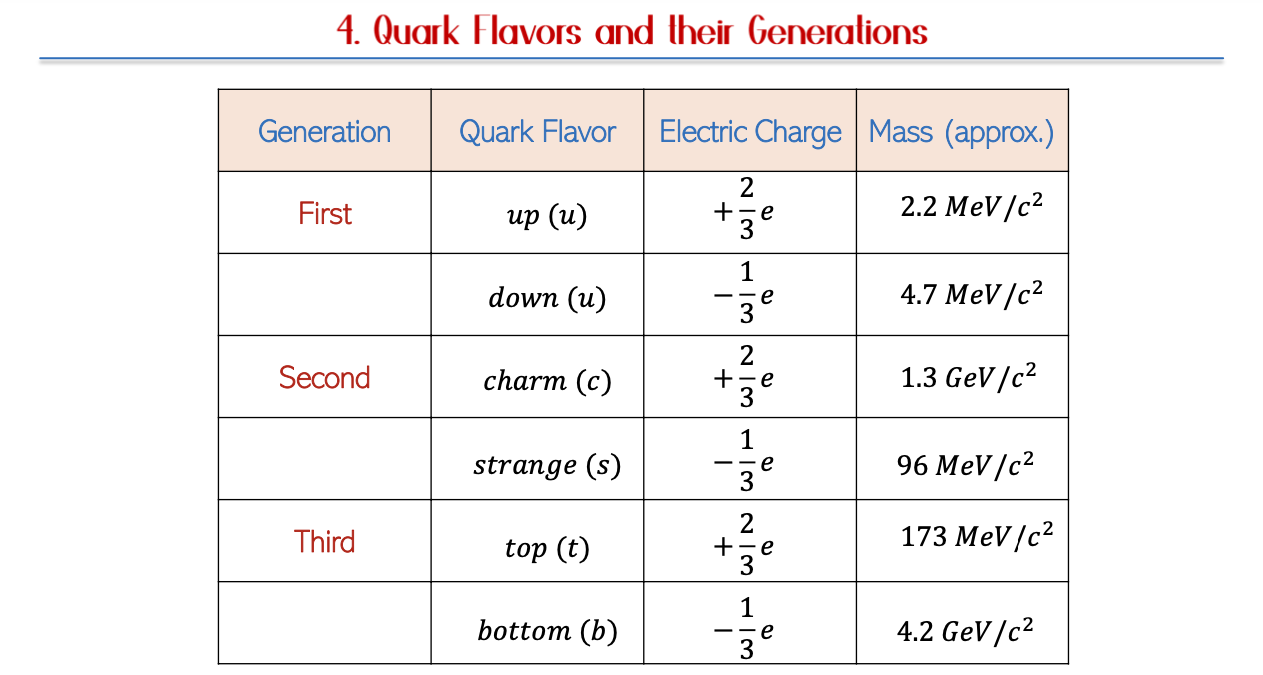
Generations of Quarks
Characteristics
The different generations of quarks possess unique masses and stability levels. The first-generation quarks, including up and down quarks, are predominant in ordinary matter. Conversely, higher generation quarks, such as top and bottom quarks, primarily appear in high-energy collisions observed in particle accelerators, indicating a hierarchy in the particle physics structure.
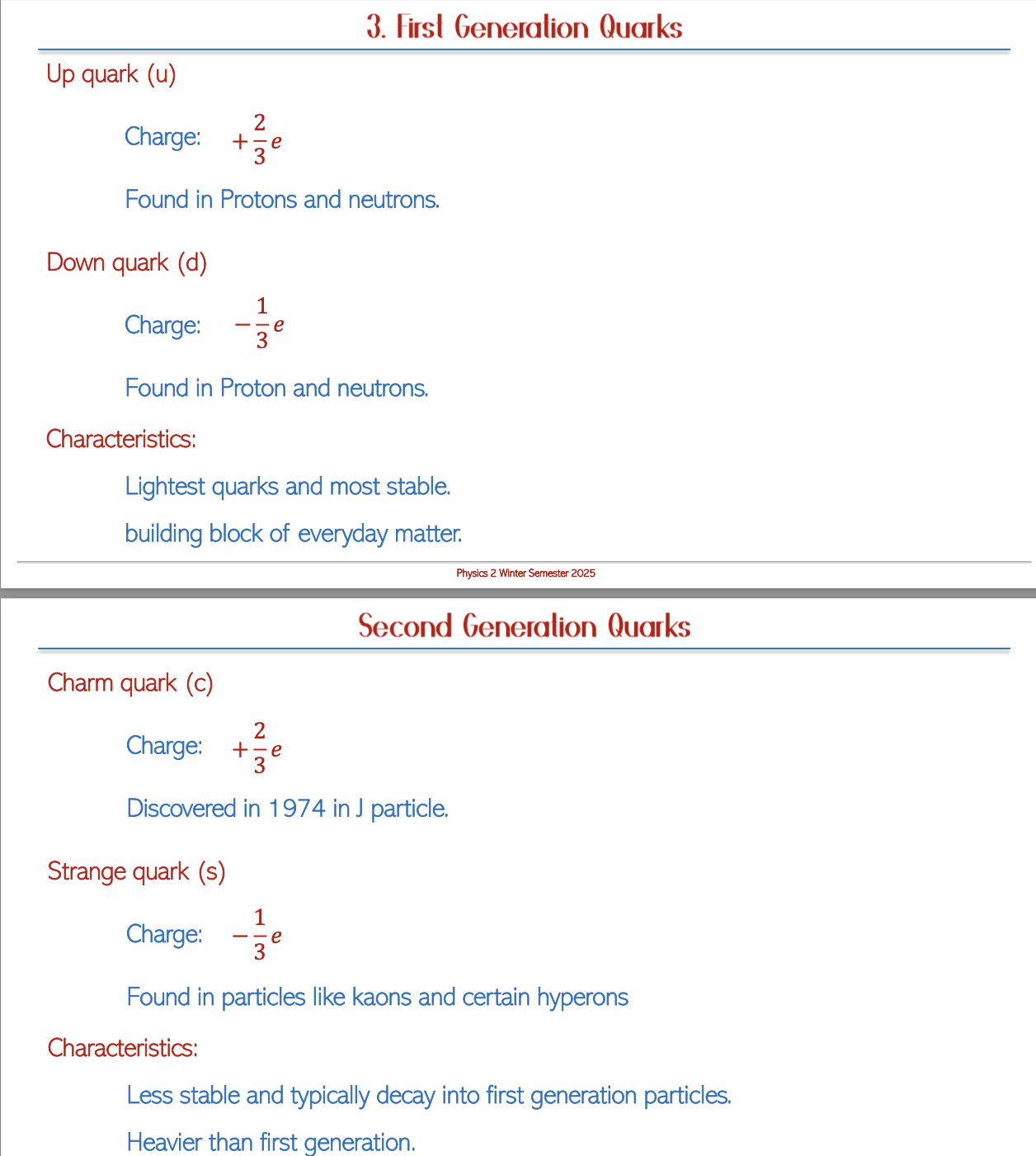


Table of Quarks per Generation

Pair Production and Annihilation
Photon Energy and Pair Production
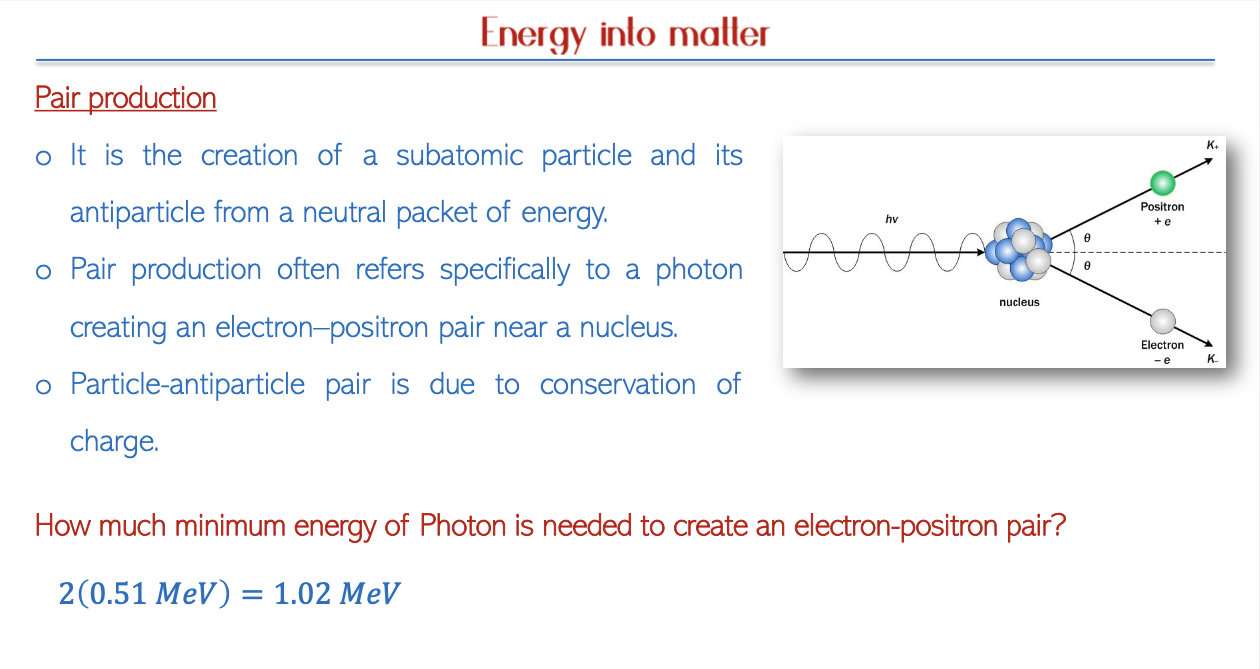
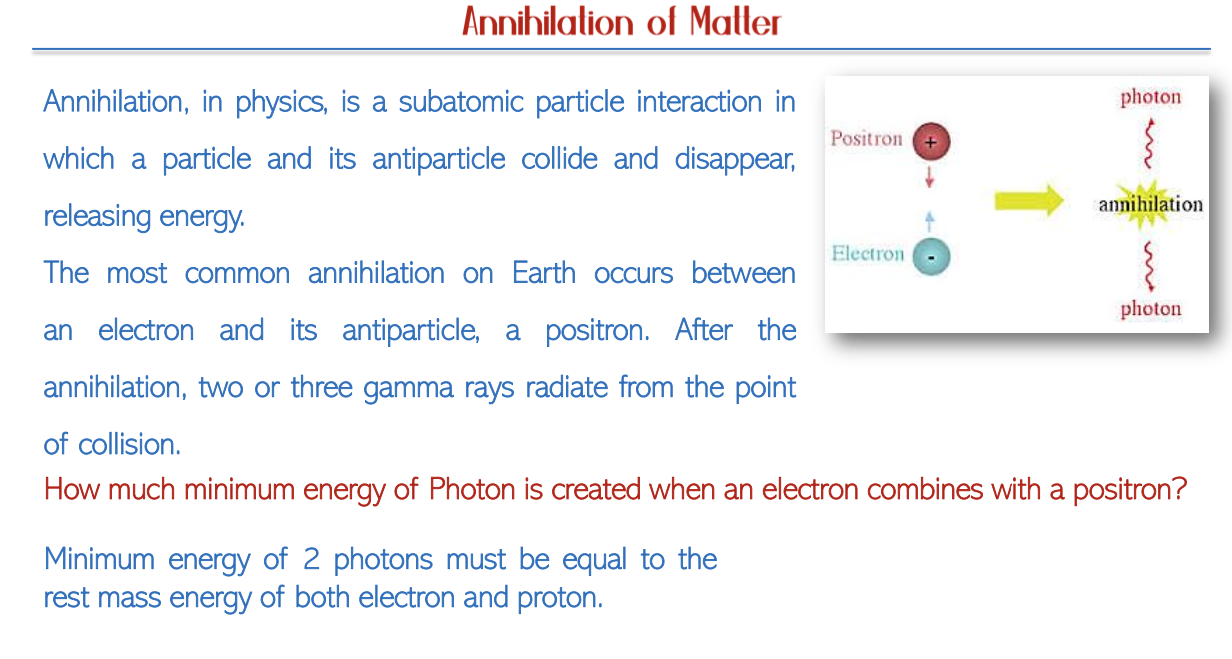
Interactions and Decay
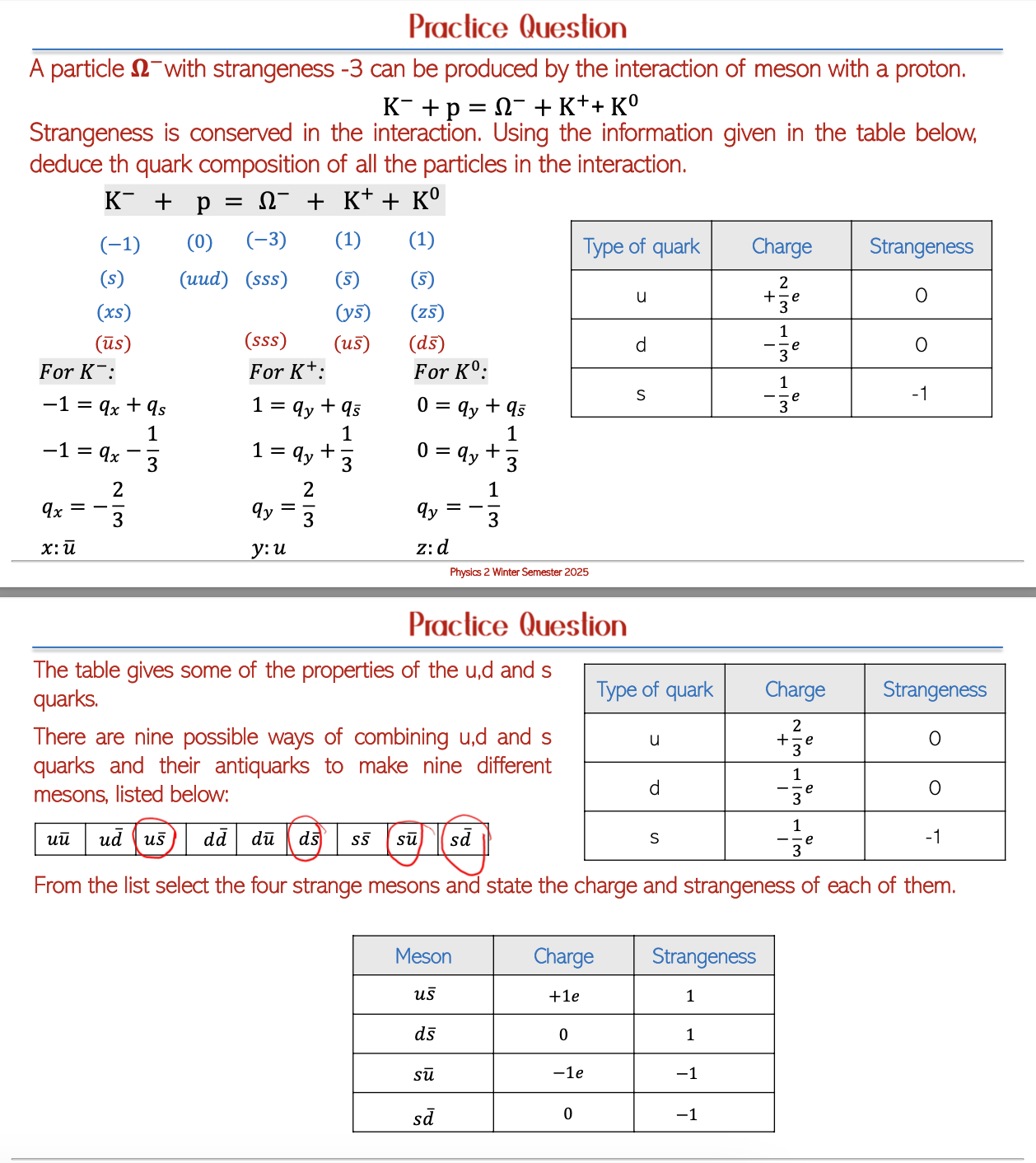
Strangeness and Quark Composition: The interaction processes in particle physics allow for calculations of quark compositions and their behaviors across various experimental settings. Fundamental properties of quarks, such as charge and strangeness, remain conserved across the interactions they undergo, providing insights into the underlying symmetries of particle physics.

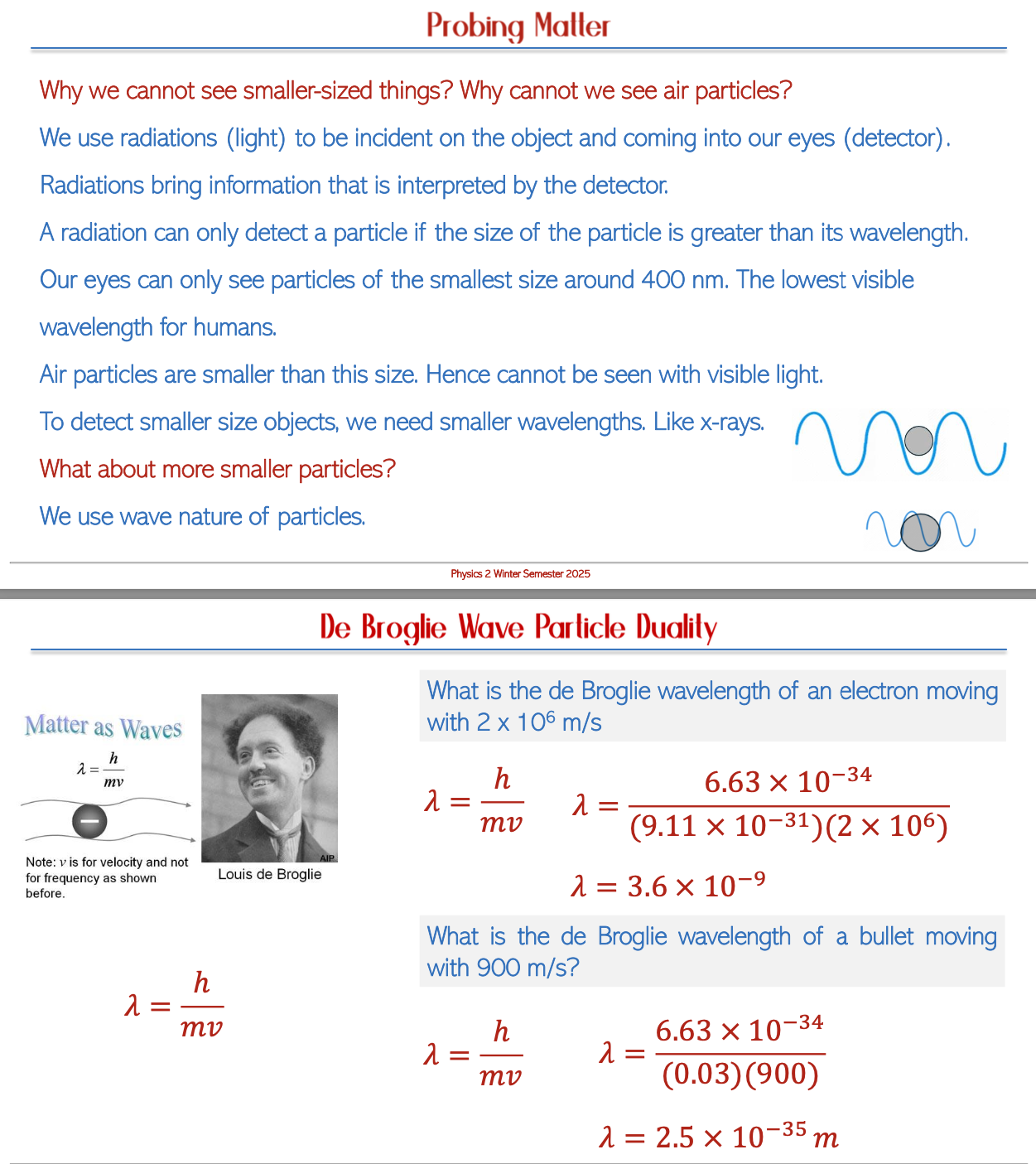
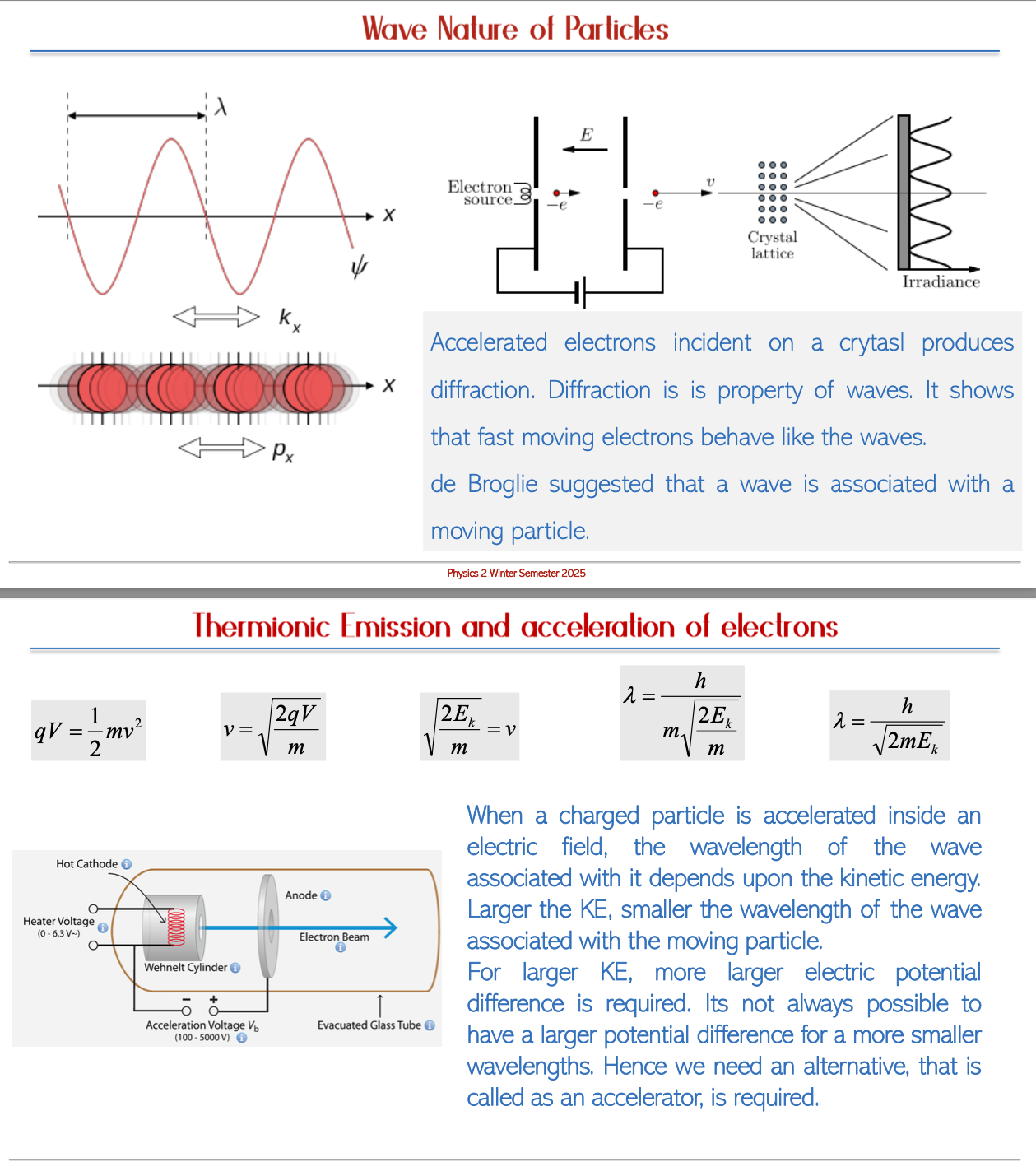

Conclusion
The study of modern physics and atomic structure offers a profound understanding of complex interactions, ranging from fundamental particles to the larger forces that govern the behavior of the universe. This knowledge is essential for advancements in various fields, including quantum mechanics, nuclear physics, and cosmology, underscoring the importance of this discipline in understanding not just our universe's composition but its underlying laws as well.