Lecture 11 - Reduction Potential Standard Electrical Potential
Oxidation/Reduction (REDOX) reactions involve changes in Oxidation States of Elements, most easily visualized as a Transfer of Electrons.
“LEO the lion says GER” = “LOSS of Electrons is Oxidation, Gain of Electrons is Reduction”
“OIL RIG” = “Oxidation Is Loss” “Reduction Is Gain”
Example of Chemical Redox Reaction:
In the reaction between iron (Fe) and oxygen (O2) to form iron oxide (Fe2O3), iron undergoes oxidation (loses electrons) while oxygen undergoes reduction (gains electrons).
4Fe + 3O2 → 2Fe2O3
Example of a biochemical redox reaction: In cellular respiration, glucose is oxidized to carbon dioxide and water, while oxygen is reduced to form water.
Example of Biogeochemical Redox: The conversion of nitrate (NO3-) to nitrogen gas (N2) by denitrifying bacteria in soil is a biogeochemical redox process.
In Biological Systems, Oxidation-Reduction reactions involve not only transfer of ELECTRONS but also transfer of HYDROGEN that has both one PROTON (H+) and one Electron (e- ).
O2 + 2H2 → 2H2O
Oxidation of H2 Gas will result in the Release of H+ and ONE Electron:
2H2 → 4H+ 4e-
This OXIDATION reactions must be coupled with REDUCTION of another SUBSTANCE that can accept Electrons:
O2 + 4e- + 4H+ → 2H2O
Thus, COUPLED reaction can be written as
O2 + 2H2 → 2H2O
“LOSS of Electrons is Oxidation -- LEO
Electron donor is the substance undergoing OXIDATION or the substance that LOSES electrons during OXIDATION
2H2 → 4H+ 4e-
This substance is also referred to as “reductant” or the “reducing agents”
Common Electron donors are
Organic Carbon Compounds
Reduced Inorganic Compounds
“Gain of Electrons is Reduction”
Electron acceptor is the substance undergoing REDUCTION or the substance that GAINS electrons during REDUCTION
O2 + 4e-+4H → 2H2O
This substance is also referred to as “Oxidant” or the “Oxidizing Agents”
The most common electron acceptors is OXYGEN, which is used in AEROBIC RESPIRATION of living organisms; plants, animals, and microorganisms.
Oxygen has a high electronegativity; thus, oxygen's high affinity for electrons makes it an ideal acceptor for low-energy electrons. With the electrons, hydrogen is added to oxygen forming water as the final product.
BUT under Oxygen FREE (absent)–environment, selected microorganisms can use alternate electron acceptors such as NO3 - (Nitrate), Fe3+ (Ferric ion) , Mn4+ (Manganese ion), and SO4 2- (Sulfate)
O2 plays two roles in the biological systems:
As an electron acceptor during respiration of biota, and as a reactant in certain biochemical reactions.
O2 is involved in chemical oxidation of several reduced chemical species
~90% of the molecular O2 present in microbial and plant cells is conserved and used for oxidative phosphorylation during respiration.
Oxygen is the most preferred electron acceptor because of its great affinity for electrons
Flooding creates two zones in wetland sol
(Colloidal suspension - A sol is a colloidal suspension made out of tiny solid particles in a continuous liquid medium.)
an oxygen-free soil zone
oxygen- containing floodwater
These conditions result in diffusion of [O2] to the anoxic sites and consumption of [O2] by the anoxic site.
The [O2] in floodwater varies both spatially and temporally and is often present at saturation levels during the photosynthetic period.
Slow [O2] diffusion rate in water and high [O2] demand by soil result in the consumption of [O2] at the soil surface and formation of thin oxidized or aerobic layer.
Thus, under poorly drained, low water table—or flooded soil conditions— two distinct soil zones are created:
an aerobic soil layer where aerobic microbes are involved in biogeochemical reactions and oxygen is used as an electron acceptor, and
an anaerobic soil layer where facultative anaerobes and obligate anaerobes function
Here, the O2 is absent, nitrate, oxides of iron and manganese, sulfate, and carbon dioxide are used as alternate electron acceptors. The interface within a given day varies depending on the photosynthetic activity in the water column and the concentration of oxygen-consuming reduced compounds.
Eutrophication of wetlands can influence the dissolved O2 content of the water column as a result of an increase in organic matter production.
This figure shows dissolved O2 concentration of water and soil columns obtained from nutrient-impacted and -unimpacted sites in the northern Everglades wetland soils.
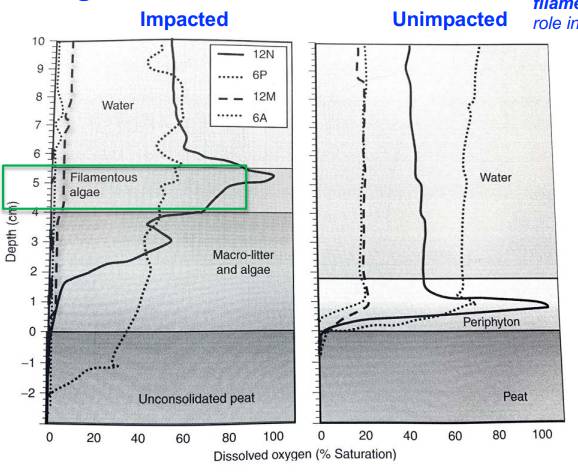
Oxygen profiles show distinct diel variations, with filamentous algae and periphyton playing a dominant role in the production and consumption of oxygen.
Periphyton is an entire community of organisms, including blue-green algae, fungi, microbes, bacteria, plant detritus, and animals that together compose the foundation of an entire ecosystem.
Oxygen production during photosynthesis occurs as follows:
CO2 + 4e- + 4H+ = CH2O + H2O
2H2O = O2 + 4e- + 4H+
CO2 + H2O = O2 + CH2O
In the eutrophic soil cores, the [O2] of the water column reached low levels during the night as a result of a high respiratory demand by microorganisms.
During the day high [O2] levels are the result of active photosynthesis mediated by algae.
Peak [O2] production is observed during midafternoon in periphyton mats when photosynthesis is much greater than respiration.
In the evening, [O2] production is slower, and [O2] diffuses into the litter layer
During the night, [O2] levels close to zero levels in the soil columns obtained from areas impacted by nutrient loading, whereas [O2] levels decrease to only 20% of saturation in the soil columns obtained from unimpacted areas
Similarly, submerged macrophytes can also alter the dissolved [O2] level of the water column with supersaturation levels during the day and negligible levels during the night.
Flooding soils for either short-term (irrigation or rainfall) or long-term (wetlands or paddy fields) results in displacement of soil [O2] —this create aerobic-anaerobic interfaces.
Dissolved [O2] present in the pore water is rapidly consumed by aerobic bacteria during their respiratory activities.
Depletion of O2 à switching of many aerobic bacteria to a facultative role and promotes the use of alternate electron acceptors.
In soils flooded for a short period, renewal of [O2] is obtained after drainage of excess pore water, whereas in poorly drained soils or soils with high energy source (histosols or mineral soils with high organic matter), [O2] demand may remain much greater than [O2] supply.
Anaerobic microsites play a significant role in nitrate losses through denitrification in upland soils.
In addition to flooding, application of oxygen demanding materials (such as organic wastes or ammoniacal fertilizers) can also result in rapid depletion of [O2] thus creating anaerobic zones.
This is more prevalent in poorly drained soils.
Reduction Potential
Reduction Potential (E0 or pE ) is the tendency of a substance undergoing oxidation to GIVE UP electrons and by the substance undergoing reduction to GAIN electrons.
These potentials are measured in reference to Standard Hydrogen Electrodes (SHE).
Substances with positive reduction potentials are usually good oxidizing agent while the substances with highly negative reduction potentials are good reducing agents.
Most of the Reduction Potentials of the various Organic and Inorganic substances are published in chemistry and biogeochemistry textbooks.
Biogeochemical cycling in wetlands refers to the movement and transformation of chemicals within these unique ecosystems. Wetlands play a crucial role in various biogeochemical processes due to their hydrological, geological, and biological characteristics. Let’s delve into some key aspects:
Nitrogen Cycle:
One of the most important biogeochemical cycles in wetlands is the nitrogen cycle.
While the potential transformations are not unique to wetlands, the dominance of anaerobic conditions in these ecosystems significantly influences nitrogen cycling.
Key processes include nitrogen fixation, nitrification, denitrification, and ammonification.
Wetlands act as both sources and sinks for nitrogen compounds, affecting water quality and nutrient availability in surrounding watersheds.
Phosphorus Cycling:
Wetlands also play a vital role in phosphorus cycling.
Among their functions are sediment trapping, nutrient removal, storage, and release.
The phosphorus cycle in wetlands impacts the transport, storage, and biological availability of phosphorus in the surrounding watershed.
Hydric Soils and Hydrophytic Vegetation:
Common diagnostic features of wetlands include hydric soils (soils that are saturated with water) and hydrophytic vegetation (plants adapted to wet conditions).
These features contribute to the unique biogeochemical processes occurring in wetlands.
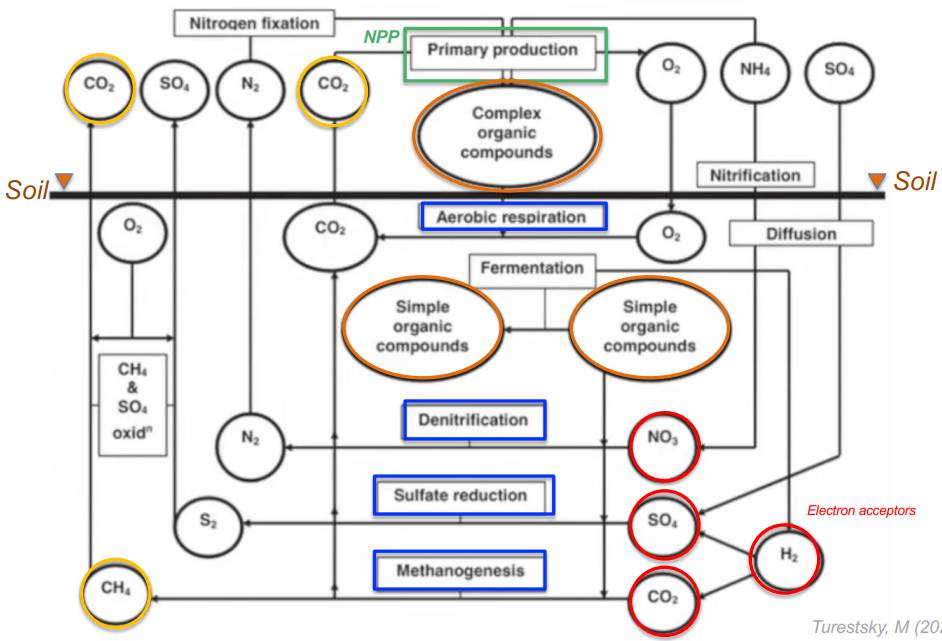
Classification of organisms based on their primary source of ENERGY reveals the metabolic diversity of microbes as compared with that of higher plants and animals.
Hydroperiod in Wetlands:
The hydroperiod refers to the duration of time that a wetland remains inundated or saturated with water.
It significantly influences wetland functions, including nutrient cycling, habitat availability, and ecosystem services.
Different wetlands exhibit varying hydroperiods, ranging from very short (only a few weeks of water retention per year) to permanent (such as lakes and ponds).
Redox Conditions in Wetland Soils:
Wetland soils are typically saturated with water, leading to anaerobic conditions (low oxygen levels).
These low-oxygen environments result in low redox values.
Redox potential (or reduction potential) describes the likelihood of an environment to receive electrons and become reduced.
The redox potential varies along the sediment column, with deeper soils having lower redox compared to surface soils.
As a result, different chemical reactions occur at different soil depths.
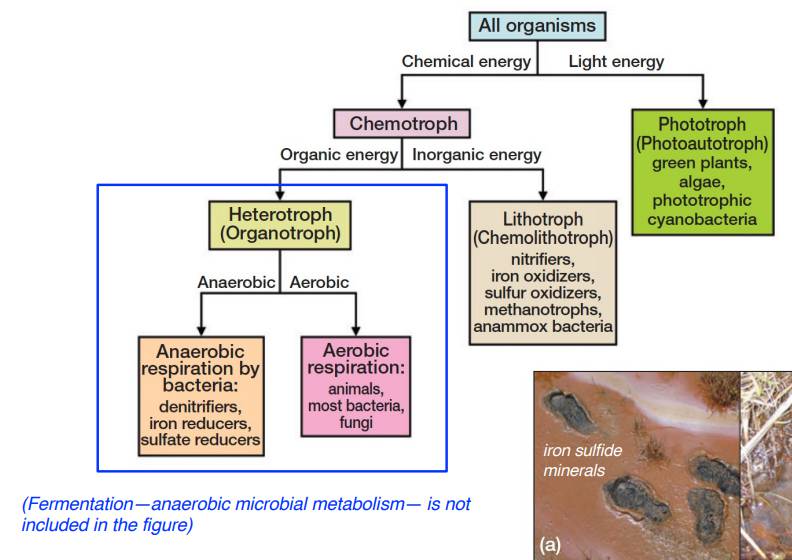
Microbial Processes and Nutrient Cycling:
Anaerobic microbial processes play a crucial role in wetland soils:
Denitrification: Bacteria convert nitrates (NO₃⁻) into molecular nitrogen (N₂).
Sulfate reduction: Reduction of sulfate ions (SO₄²⁻) produces hydrogen sulfide (H₂S).
Methanogenesis: Anaerobic bacteria generate methane (CH₄).
Changes in the oxidation state of iron and manganese also occur due to microbial activity.
Incomplete decomposition under anaerobic conditions leads to the accumulation of organic carbon in wetland soils.
Importance of Hydroperiod:
The hydroperiod directly influences which amphibian species can successfully breed in a wetland.
Understanding wetland hydroperiod is crucial for effective management decisions to conserve amphibian breeding habitat.
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. Let’s break it down:
Oxidation: In this process, some atoms lose electrons. It’s like they’re getting “stripped” of their electrons. This is called oxidation.
Reduction: On the other hand, some atoms gain electrons. They’re essentially receiving electrons. This is called reduction.
Simultaneous Process: The fascinating thing about redox reactions is that both oxidation and reduction occur simultaneously. Hence, the term “redox.”
A half reaction (or half-cell reaction) is a fundamental concept in redox chemistry. Let’s break it down:
A half reaction represents either an oxidation or a reduction process separately within an overall redox reaction.
In other words, it focuses on the change in oxidation states of individual substances involved in the redox reaction.
Measuring REDOX : ELECTRON AND REDOX REACTIONS
All Redox reactions, such as this reaction:
2Fe3+ + H2 < - > 2Fe2+ + 2H+
can be broken down to into a Reduction HALF-REACTION, in this case:
2Fe3+ + 2e- < - > 2Fe2+
[ for one electron: Fe3+ + e- < - > Fe2+ ]
Thus the Oxidation HALF-REACTION is
H2 < - > 2H+ + 2e
Adding these two half reactions together gives the overall reaction
2Fe3+ + H2 < - > 2Fe2+ + 2H+
The addition of an oxidation half reaction and a reduction half-reaction—each expressed for the same number of electrons so that the electrons CANCEL on both sides of the arrows—give a WHOLE redox reaction.
The equilibrium of a REDOX reaction, i.e., the degree to which the reaction as written tends to lie to the RIGHT or LEFT, can be deduced from information about its constituent half-reactions.
OIL → Oxidation: Loss of electrons
RIG → Reduction: Gain of electrons
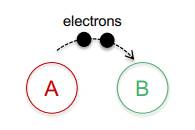 A loses electrons B gains electrons
A loses electrons B gains electrons
A is oxidized B is reduced
A gives electrons to B, reducing it B takes electrons from A, oxidizing it
A is the reducing agent B is the oxidizing agent
The “thing” that is oxidized is the reducing agent.
The “thing” that is reduced is the oxidizing agent.
Oxidation number, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
Each atom that participates in an oxidation-reduction reaction is assigned an oxidation number that reflects is ability to acquire, donate or share electrons
Oxidation: Loss of electrons Reduction: Gain of electrons
oxidation number increases oxidation number decreases
Photosynthesis
Green plants convert water and carbon dioxide into carbohydrates, defined as photosynthesis. The reaction is given as 6CO2 + 6H2O → C6H12O6 + 6O2.
Carbon dioxide is reduced to carbohydrates while the water gets oxidized to oxygen; hence, it is a Redox reaction. The energy is provided by the sunlight for this reaction. This reaction is a source of food for animals and plants.
Oxidation/Reduction reactions are significant in water
Bacterially mediated OXIDATION of biomass depletes Oxygen
{CH2O} + O2 → CO2 + H2O
Bacterially mediated REDUCTION of solid Iron Oxides and Hydroxides puts soluble iron in water
Fe(OH)3(s) + 3H+ + e- → Fe2+(aq) + 3H2O
Microbially mediated OXIDATION of Ammonium Nitrogen (NH4+) produces Nitrate (NO3-), which can be assimilated by Algae
NH4+ + 2O2 → NO3- + 2H+ + H2O
Nitrification
Two important points regarding Oxidation/Reduction in water:
These reactions are mediated by Microorganisms
Close relationship with acid-base, analogies between e- and H+.
Whereas the activity of the H+ ion is used to express the extent to which water is ACIDIC OR BASIC, the activity of the electron, e- is used to express the degree to which an aquatic medium is OXIDIZING or REDUCING.
Water with a HIGH hydrogen-ion activity, such as from runoff from “acid rain” is ACIDIC. By analogy, water with a high electron activity, such as that in the Anoxic Digester of a sewage treatment plant or Anoxic wetland soil, is said to be REDUCING.
Water with a low hydrogen-ion activity (high OH- ) activity—for example a landfill leachate contaminated with waste sodium hydroxide— is BASIC.
Water with a low electron (e- ) activity—highly chlorinated water , or a not flooded wetland – is said to be OXIDIZING.
Actually, neither free electrons nor free H+ ions as such are found dissolved in aquatic solutions or wetland pore waters e- and H+ are always strongly associated with SOLVENTS or SOLUTES.
“A solvent (from the Latin solvō, "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid”
However, the concept of ELECTRON ACTIVITY—like that of Hydrogen-ion activity—remains a very useful one to aquatic/wetland chemists and ecologists.
Many species in water undergo exchange of BOTH electrons and H+ ions.
For example, ACID MINE water contains the hydrated iron (III), Fe(H2O)6 3+ , which readily loses H+ ion and contributes ACIDITY to the medium: Fe(H2O)6 3+ < - > Fe(H2O)5OH2+ + H+
The same ION accepts an electron to give IRON (II): Fe(H2O)6 3+ + e- < - > Fe(H2O)6OH2+
Generally, the transfer of electrons in a REDOX REACTION is accompanied by H+ ion transfer, and there is a close relationship between REDOX and Acid-Base processes.
If IRON (II) loses an electron at pH 7, three hydrogen ions are also lost to form highly INSOLUBLE iron (II) hydroxide-- an insoluble gelatinous solid
Fe(H2O)6 2+ <- > e- + Fe(OH)3 (s) + 3H2O + 3H+
Measuring REDOX : ELECTRON AND REDOX REACTIONS
All redox reactions involve changes in the oxidation state of some of the species that take part in the reaction. For example, assume a solution containing iron (II) and iron (III) that is sufficiently ACIDIC to prevent precipitation of SOLID Fe(OH)3. This solution might be Acid Mine water or a Steel pickling liquor waste.
Suppose the solution is treated with with elemental hydrogen gas over a suitable catalyst to bring about the reduction of IRON (III) to IRON (II): 2Fe3+ + H2 < - > 2Fe2+ + 2H+
This reaction is reversible — for normal concentrations of reaction participants— this reaction goes to the right.
2Fe3+ + H2 < - » 2Fe2+ + 2H+
As the reaction goes to the right, the HYDROGEN is OXIDIZED as it changes from an OXIDATIONS STATE (number) of 0 in elemental H2 to a higher oxidation number of +1 in H+.
The Oxidation state of IRON goes from +3 in Fe3+ to +2 in Fe2+. The Oxidation number of IRON decreases, meaning that it is now REDUCED.
“LOSS of Electrons is Oxidation, Gain of Electrons is Reduction”
Substantial changes in the distribution of chemical species in water--resulting from REDOX reactions--are vitally important to aquatic organism and have a tremendous influence on water quality.
These type of systems are assumed to be at equilibrium, a state almost never achieved in any real natural water or wastewater.
Most REAL aquatic and wetland systems are DYNAMIC systems that may approach a STEADY STATE, rather than EQUILIBRIUM
Regulators of wetlands soil REDOX conditions
Water table fluctuations
Activates of electron acceptors
Activities of electron donors
Microbial populations dynamics
Temperature
pH
The transport of O2 in a well-drained soil is sufficient to supply the oxygen needed to support the growth of microbial and plant populations
Soil remain at high Eh level (>300 mv) in the presence of O2. Standard Electrical Potentials (Eh) are expressed as per mol of electrons transferred.
Gas exchange in the these soils prevents the depletion of O2 and excessive accumulation of CO2 in the soil profile.
O2 Flux is always from the atmosphere to the soils because the [O2] in soil pores is lower that that in the atmosphere.
CO2 Flux is always from the soil to the atmosphere because the [CO2] in pore waters is typically higher here.
Heterotrophic respiration of organic matter can be either aerobic (involving oxygen) or anaerobic (not requiring oxygen). Both forms of respiration are oxidation– reduction reactions, in which simple organic carbon compounds are combined with electron (e-) acceptors to yield oxidized carbon (CO2), reduced products (H2O in the case of aerobic respiration), and ENERGY.
For example, the process of respiratory Denitrification (N2 gas production) is a form of anaerobic respiration in which nitrate (NO3) serves as the alternate e- acceptor.
Several substances can act as e- acceptors in anaerobic respiration and depending on the e- acceptor and its ultimate product, variable amounts of energy are produced.
Some common e- acceptors are listed in order from highest to lowest efficiency of energy yield; Those microbes performing the more efficient reactions tend to outcompete others for labile organic matter.