Inorganic analysis
LV content:
1.Flame Tests for Cations:
A cation is any positively charged ion.
Some metal cations can be identified by the colour they produce in a flame.
Method:
Dip the end of a nichrome wire into hydrochloric acid (HCl), then into the solid sample.
Place the wire into a roaring blue flame and observe the flame colour.
Compare the colour to known results to identify the metal ion.
(The wire is often cleaned first by dipping it in acid and placing it in the flame to remove impurities.)
NB: When magnesium metal is burned in oxygen it produces a bright white light. This is not the same as testing for magnesium cations. There is no flame colour produced by Mg2+
Sodium Hydroxide Tests for Cations:
Many metal cations form insoluble solids (precipitates) when sodium hydroxide solution is added.
The colour of the precipitate helps identify the metal ion.
Method:
Add sodium hydroxide solution (NaOH(aq)) to a solution of the substance being tested.
Observe the colour of the precipitate formed to determine the metal ion present.
To test for ammonium ions, sodium hydroxide solution is added to the solid or the aqueous substance to be tested and heated. Ammonia gas is produced whihc turns red litmus paper blue.
Tests for Anions:
A negatively charged ion is called an anion.
The table below shows the test results for three specific anions:
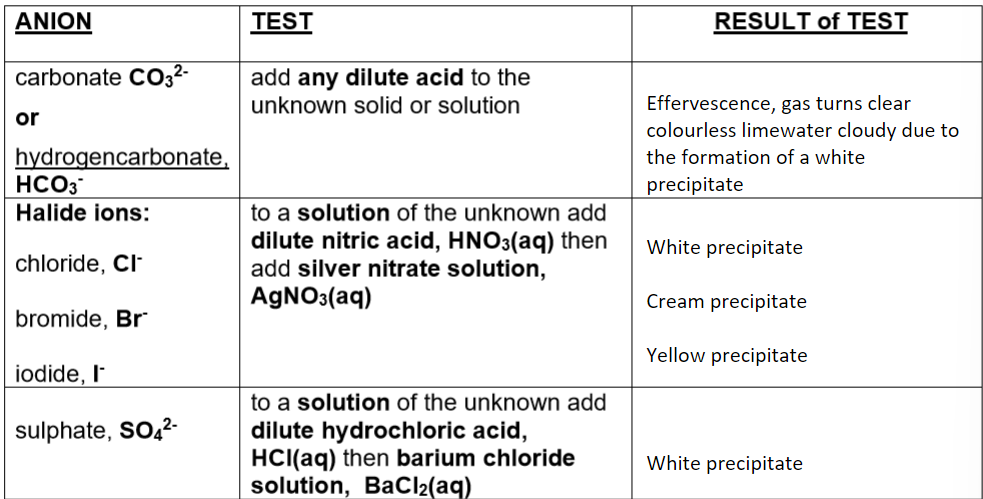
NB: If a solid is heated and CO2 and H2O are produced, the solid has to be a hydrogen carbonate, HCO3.
Experiments:
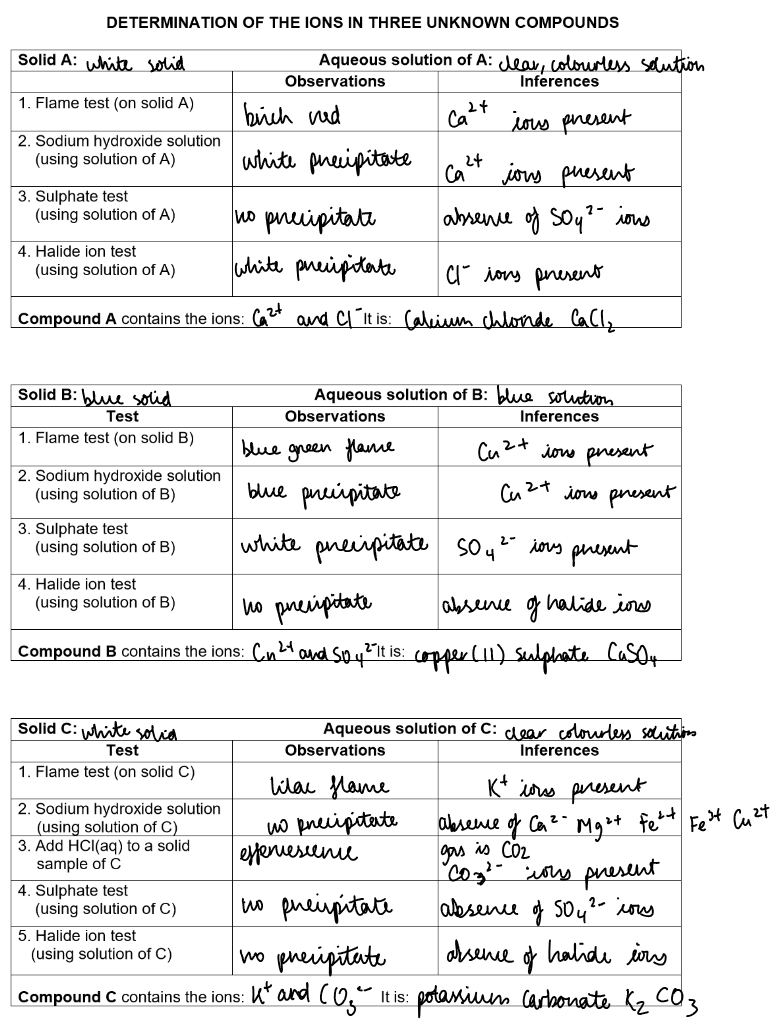
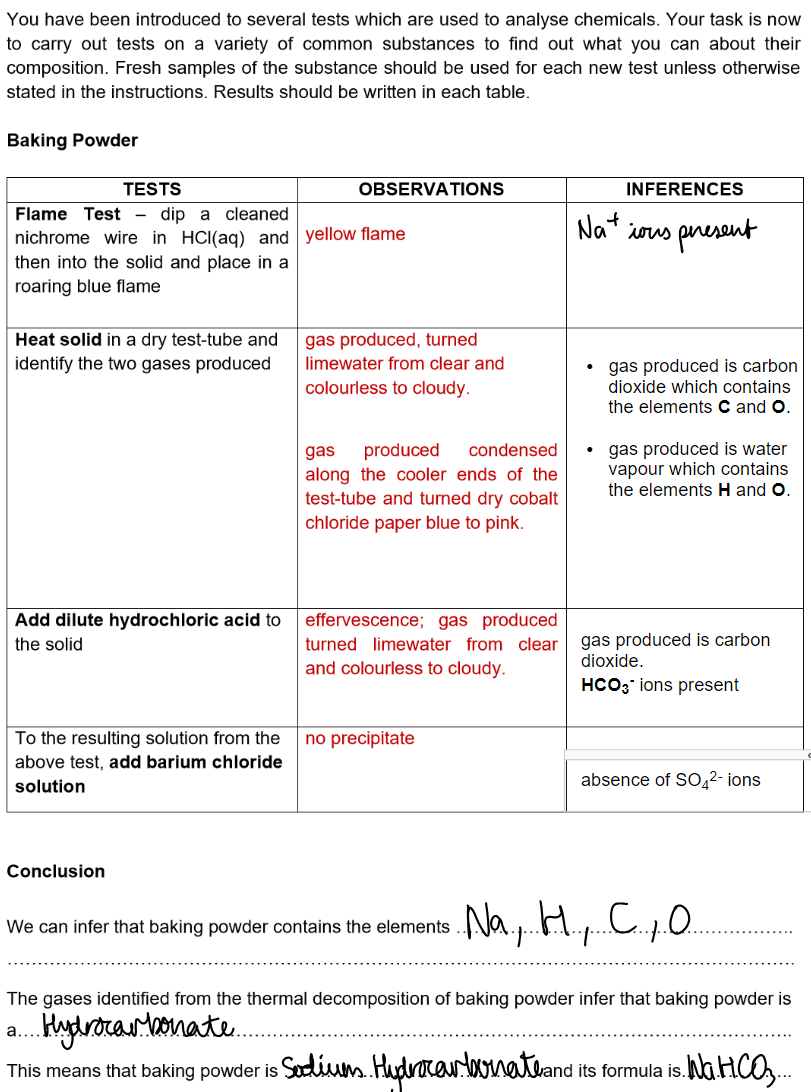
Analysis practicals:
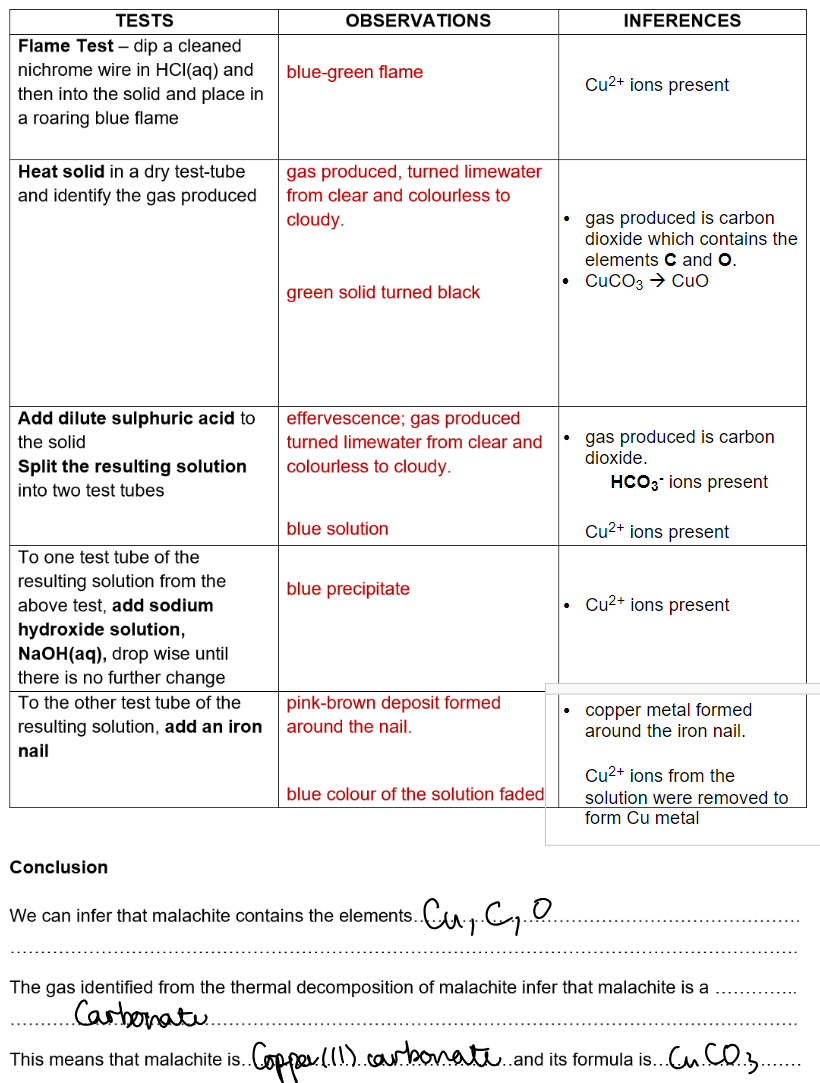
Malachite:
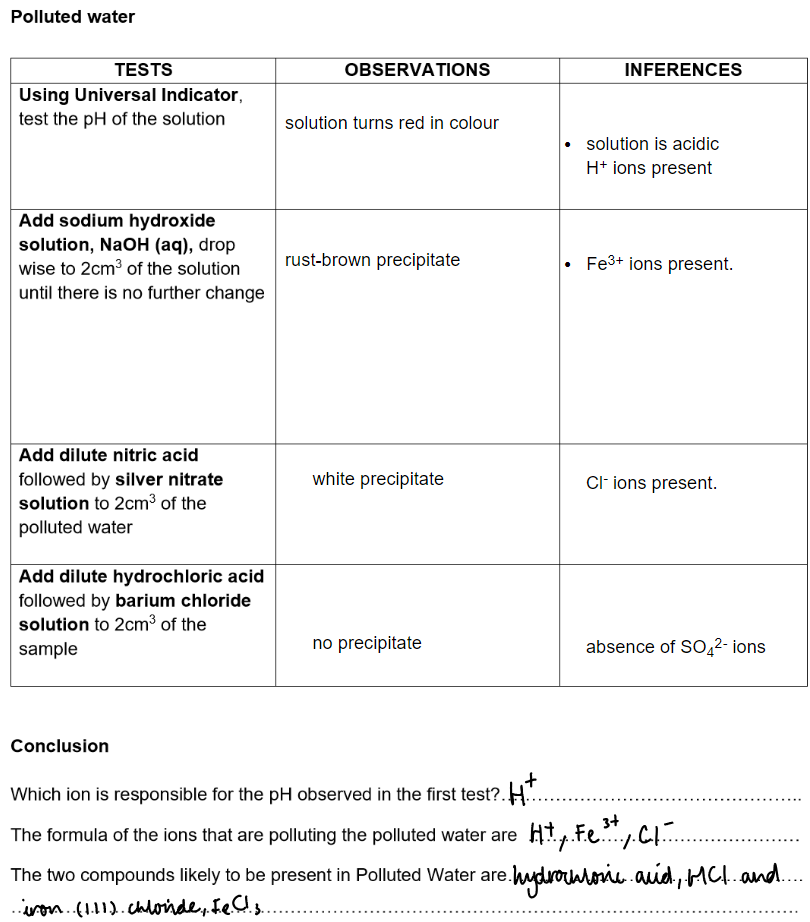
Polluted water:
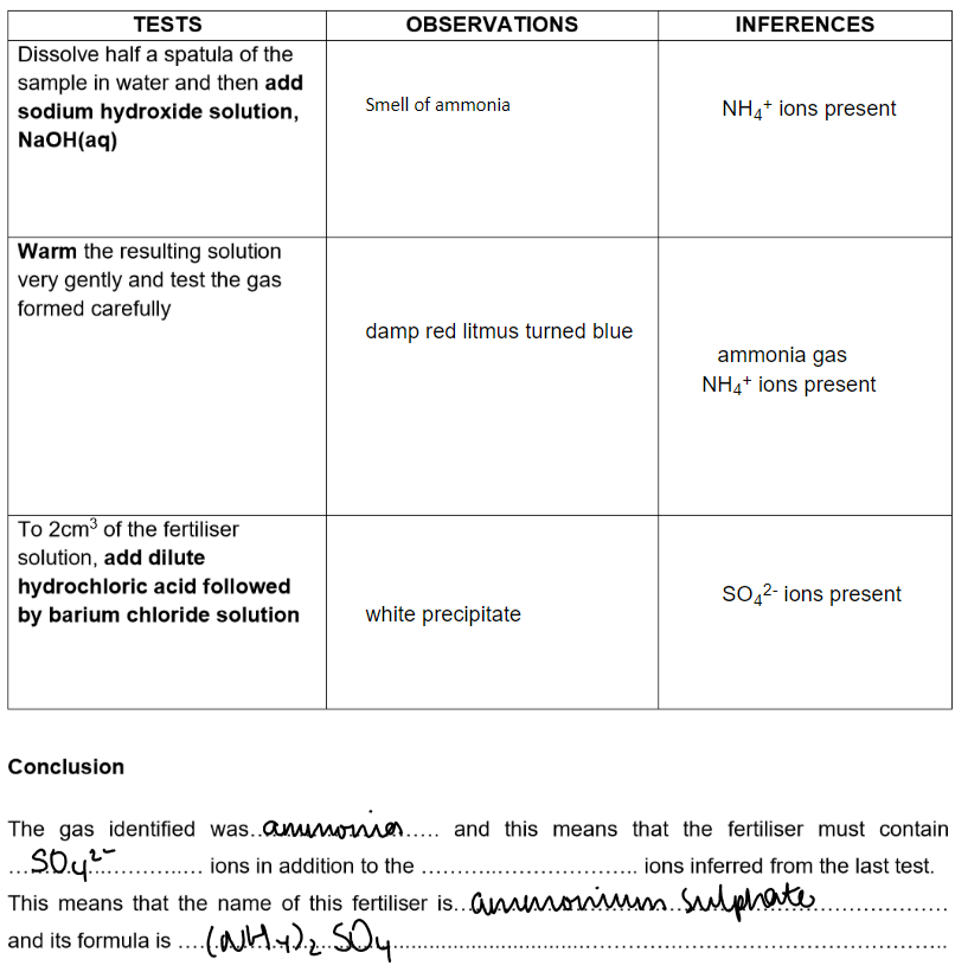
Fertiliser:
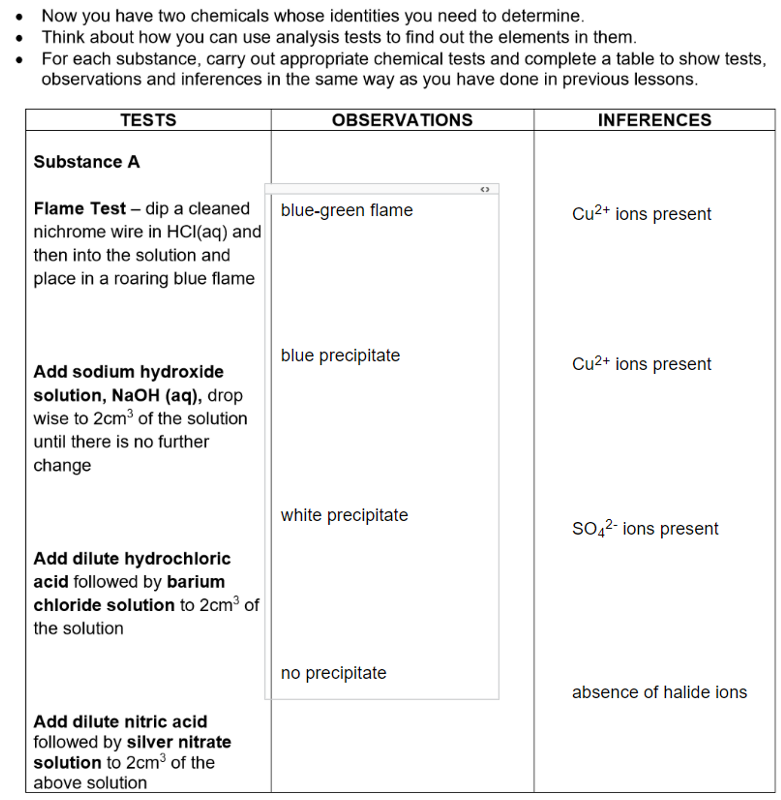
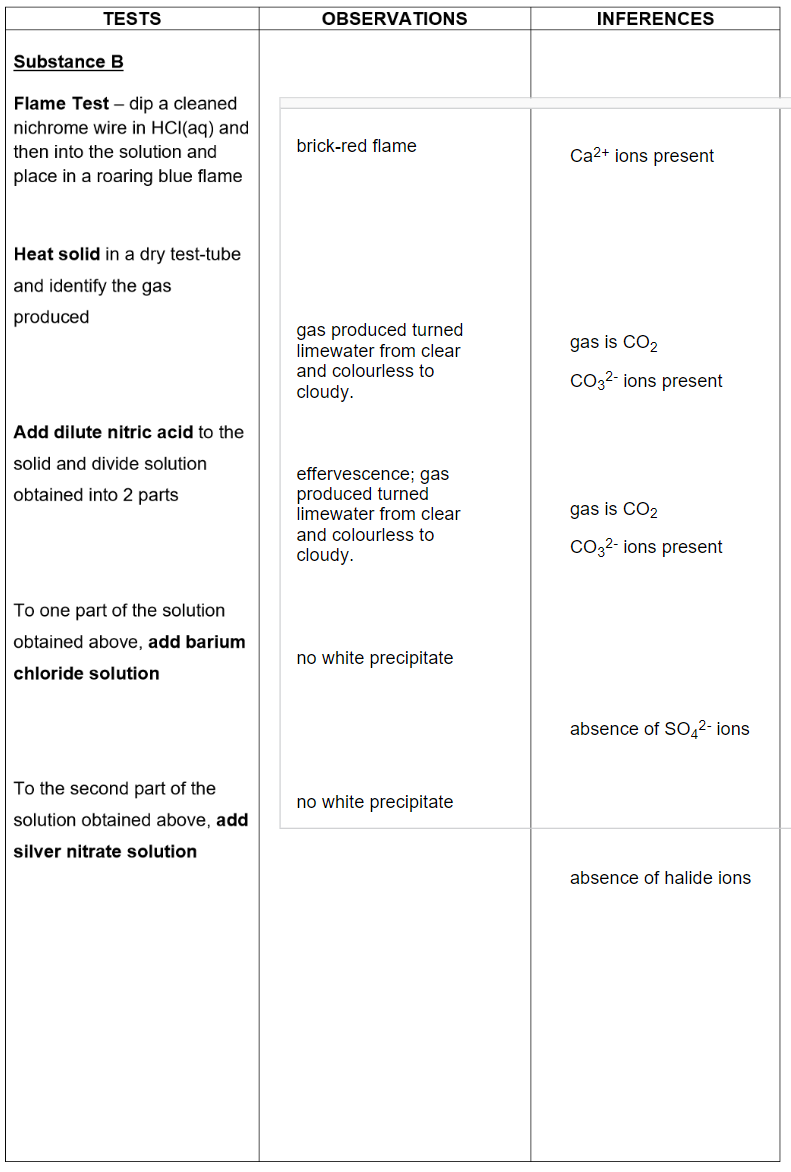
Analysis of rock mixture:
AIM
You have been given a sample of rock which is a mixture of two different solids. One of the solids is soluble in water and the other one is insoluble in water. Your task is to separate the mixture and to analyse the two solids to find out which elements they contain.
1.
(a) Observe the rock mixture and write down its appearance.
a mixture of blue and white solid
(b) Does the appearance give you any clues about the identity of any elements in the rock?
blue → copper(II) sulphate crystals; white → marble chips
2.
(a) List the names of some different methods of separation.
filtration, decanting, centrifuging
simple distillation, fractional distillation
use of a separating funnel, magnets, chromatography
(b) It states above that one of the components of the mixture is water-soluble and the other is insoluble. Using this information, describe practical details which you would use to separate the two solids.
Add water to the rock mixture and stir
Filter the mixture using a filter funnel and filter paper
The white solid is the residue and the blue solution is the filtrate
Wash the residue with a small amount of water to dissolve any water-
soluble impurities
3.
(a) Describe the appearance of the soluble substance. Let this be called substance A.
blue solution
(b) Describe the appearance of the insoluble substance. Let this be called substance B.
white solid
4. Once you have successfully separated the two substances, it is advisable to wash the insoluble solid with a little distilled water. Why is this?
to dissolve any water soluble impurities contaminating it.
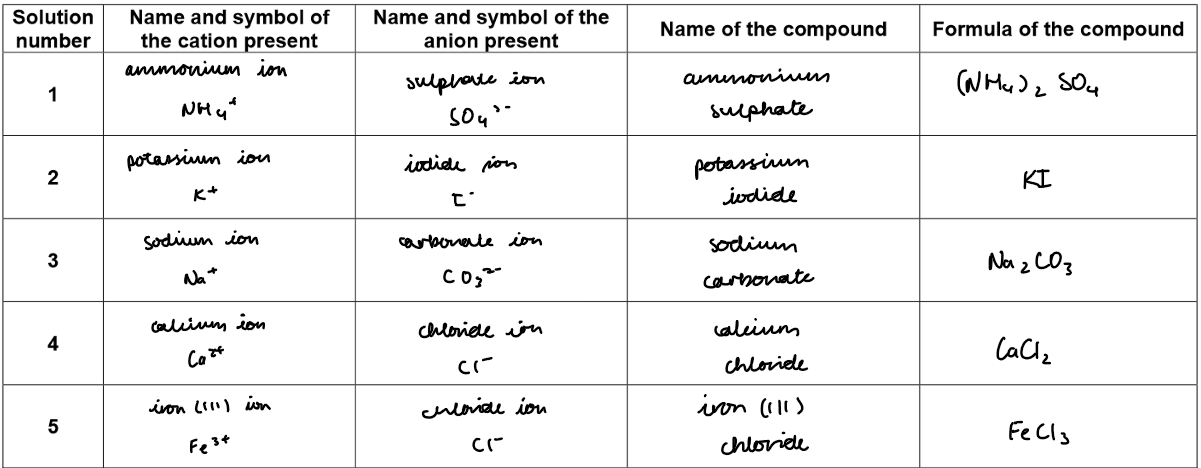
5. From the results:
Substance A contained the elements Cu²⁺ and SO₄²⁻
and is likely to be the compound copper(II) sulphate
formula CuSO₄
Substance B contained the elements Ca²⁺ and CO₃²⁻
and is likely to be the compound calcium carbonate
formula CaCO₃
6.
(a) Compound B is widely used in building and as a starting material for industrial processes. It can exist in three different forms. What are the names of these?
chalk, marble and limestone
(b) What are some of the major uses for substance B.
building material, cement and glass manufacture, neutralises acidic soil
UV content:
Demo Gas Tests and Chemical Testing for Ions
• Safety: Demonstrations are carried out by your teacher.
• Follow all safety instructions carefully and wear goggles if advised.
Oxygen
• Test: Glowing splint
• Observation: Splint relights
• Notes: Same density as air; almost insoluble; collect over water or in a gas syringe
Hydrogen
• Test: Lighted splint
• Observation: Pops with small explosion
• Notes: Less dense than air, almost insoluble; collect upwards or over water
• Equation: 2H₂ + O₂ → 2H₂O
Carbon Dioxide
• Test: Bubble through limewater
• Observation: Limewater turns milky
• Notes: Denser than air, slightly soluble; collect downwards
• Equation: Ca(OH)₂ + CO₂ → CaCO₃ + H₂O
Chlorine
• Test: Damp blue litmus paper
• Observation: Paper bleaches white
• Notes: Green gas, dense, very soluble; collect by downward delivery
Ammonia
• Tests: Damp red litmus paper; add HCl(g)
• Observation: Paper turns blue; white smoke with HCl
• Notes: Less dense than air; alkaline; very soluble
• Equation: NH₃ + HCl → NH₄Cl (white smoke)
Water
• Tests: Anhydrous CuSO₄(s); cobalt(II) chloride paper
• Observation: CuSO₄ turns blue; CoCl₂ paper turns pink
• Notes: Confirms presence of water, not purity
• Equation: CuSO₄ + 5H₂O → CuSO₄·5H₂O
Hydrogen Chloride
• Tests: Damp blue litmus paper; add NH₃(g)
• Observation: Paper turns red; white smoke with NH₃
• Notes: Dense acidic gas; very soluble; forms steamy fumes in air
• Equation: HCl + NH₃ → NH₄Cl (white smoke)
Alkenes
• Test: Bromine water
• Observation: Orange solution decolourises
• Notes: Confirms presence of a C=C bond (unsaturated hydrocarbon)
Physical Tests for Pure Water
• Boiling point – 100°C
• Freezing point – 0°C
• Density – 1.0 g cm⁻³ (mass = volume in g and cm³)
Cation Tests
1) Flame Tests
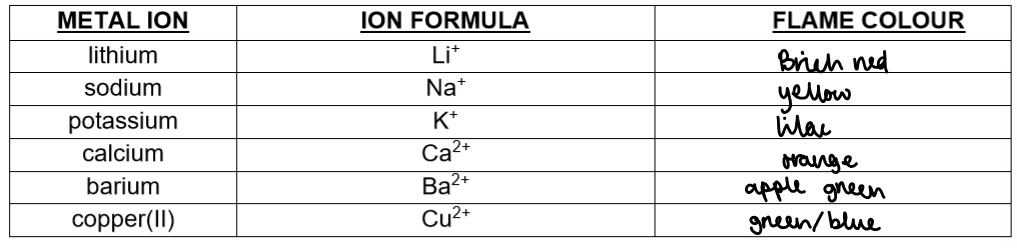
Lithium (Li⁺)
Test: Dip a nichrome wire into hydrochloric acid, then into the solid compound, and place it in a non-luminous Bunsen flame.
Observation: Crimson red flame
Sodium (Na⁺)
Test: Same as above
Observation: Yellow/orange flame
Potassium (K⁺)
Test: Same as above
Observation: Lilac flame
Calcium (Ca²⁺)
Test: Same as above
Observation: Brick red/orange-red flame
Copper (Cu²⁺)
Test: Same as above
Observation: Blue-green flame
Sodium Hydroxide Solution Test
Purpose: Used when a positive ion does not give a distinctive flame colour. Requires a solution of the compound.
Ammonium ions (NH₄⁺)
Test: Add sodium hydroxide solution and warm
Observation: No precipitate; pungent smell; NH₃ gas released which turns damp red litmus blue
Notes / Equations:
NH₄NO₃(s) + NaOH(aq) → NH₃(g) + NaNO₃(aq) + H₂O(l)
NH₄⁺(aq) + OH⁻(aq) → NH₃(g) + H₂O(l)
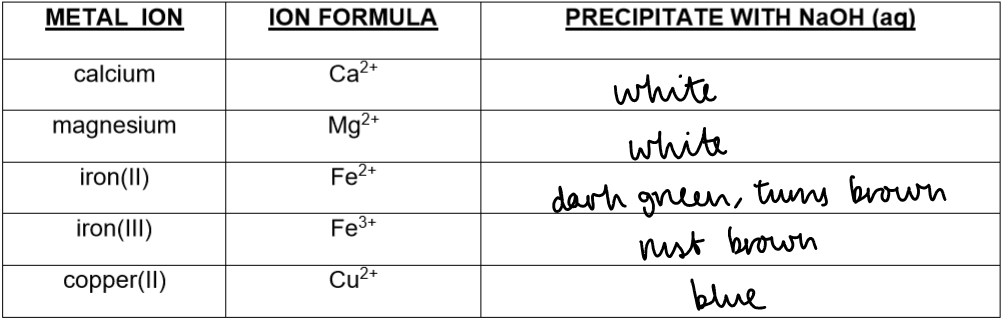
Copper(II) ions (Cu²⁺)
Test: Add sodium hydroxide solution
Observation: Light blue precipitate
Notes / Equation: CuSO₄(aq) + 2NaOH(aq) → Cu(OH)₂(s) + Na₂SO₄(aq)
Iron(II) ions (Fe²⁺)
Test: Add sodium hydroxide solution
Observation: Green precipitate
Notes / Equation: FeSO₄(aq) + 2NaOH(aq) → Fe(OH)₂(s) + Na₂SO₄(aq)
Iron(III) ions (Fe³⁺)
Test: Add sodium hydroxide solution
Observation: Brown precipitate
Notes / Equation: FeCl₃(aq) + 3NaOH(aq) → Fe(OH)₃(s) + 3NaCl(aq)
Calcium ions (Ca²⁺)
Test: Add sodium hydroxide solution
Observation: White precipitate
Notes / Equation: Ca(NO₃)₂(aq) + 2NaOH(aq) → Ca(OH)₂(s) + 2NaNO₃(aq)
Anion Tests
Carbonate ions (CO₃²⁻)
Test: Add an acid
Observation: Effervescence; CO₂ gas turns lime water cloudy (white ppt)
Notes / Equations:
Na₂CO₃(s) + 2HCl(aq) → 2NaCl(aq) + CO₂(g) + H₂O(l)
CO₂(g) + Ca(OH)₂(aq) → CaCO₃(s) + H₂O(l)
Sulphate ions (SO₄²⁻)
Test: Acidify with dilute HCl, then add BaCl₂(aq)
Observation: White precipitate forms
Notes / Equations:
HCl removes CO₃²⁻ (prevents unwanted precipitate)
BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq)
Ba²⁺(aq) + SO₄²⁻(aq) → BaSO₄(s)
Chloride ions (Cl⁻)
Test: Acidify with dilute HNO₃, then add AgNO₃(aq)
Observation: White precipitate
Notes / Equation: AgNO₃(aq) + KCl(aq) → AgCl(s) + KNO₃(aq)
Bromide ions (Br⁻)
Test: Acidify with dilute HNO₃, then add AgNO₃(aq)
Observation: Cream precipitate
Notes / Equation: AgNO₃(aq) + KBr(aq) → AgBr(s) + KNO₃(aq)
Iodide ions (I⁻)
Test: Acidify with dilute HNO₃, then add AgNO₃(aq)
Observation: Yellow precipitate
Notes / Equation: AgNO₃(aq) + KI(aq) → AgI(s) + KNO₃(aq)
Hydroxide ions (OH⁻)
Test: Add universal indicator or warm with NH₄⁺
Observation: Dark green, blue or violet; damp red litmus turns blue
Notes / Equations:
NH₄NO₃(aq) + NaOH(aq) → NH₃(g) + NaNO₃(aq) + H₂O(l)
NH₄⁺(aq) + OH⁻(aq) → NH₃(g) + H₂O(l)
Hydrogen ions (H⁺)
Test: Add universal indicator, reactive metal, or carbonate
Observation: Red, orange or yellow; effervescence
Notes / Equations:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Na₂CO₃(s) + 2HCl(aq) → 2NaCl(aq) + CO₂(g) + H₂O(l)
Thermal Decomposition of Metal Carbonates
Copper(II) carbonate: Heat → green to black
Zinc carbonate: Heat → white to yellow (returns to white on cooling)
Solubility of Salts
Soluble:
All sodium, potassium, and ammonium salts
All nitrates
All chlorides (except AgCl and PbCl₂)
All sulphates (except BaSO₄, CaSO₄, PbSO₄, and Ag₂SO₄)
Insoluble:
All carbonates (except Na⁺, K⁺, NH₄⁺)
All hydroxides (except Na⁺, K⁺, NH₄⁺; these are not salts)
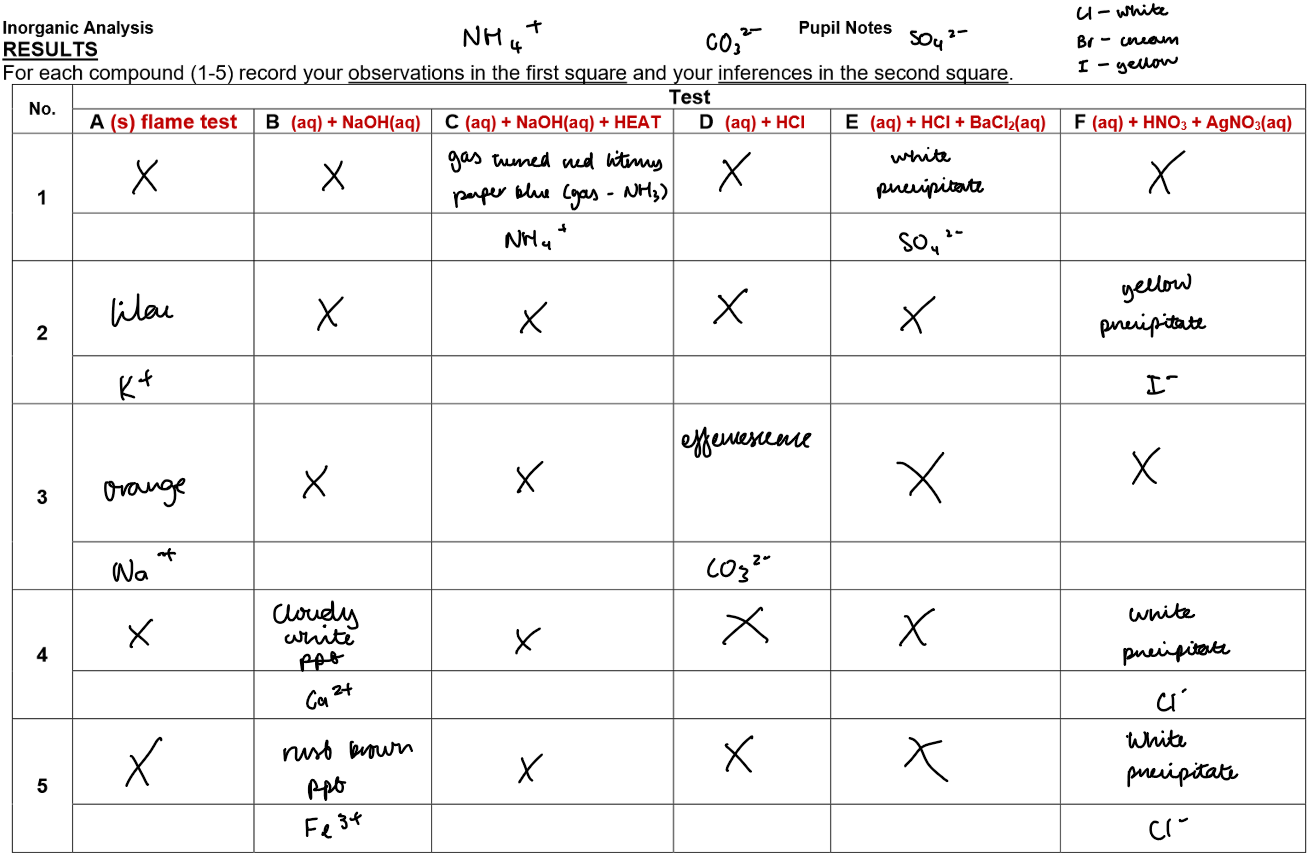
Identification of Unknown Salts
Test A: Flame Test for Li⁺, Na⁺, K⁺, Ca²⁺ (solid samples)
Clean nichrome wire in HCl, place in roaring flame.
Dip into acid again, then into unknown solid, place in flame.
Observation: Note flame colour to identify cation.
Test B: Sodium Hydroxide Test for Cu²⁺, Fe²⁺, Fe³⁺, Ca²⁺ (aqueous solution)
Place 1 cm depth of unknown solution in a test tube.
Add NaOH dropwise, then until ~2 cm³ or no further change.
Shake gently; note precipitate colour or changes.
Test C: NH₄⁺ Test with NaOH and Heating – CARE! (only solution 1)
Place 1 cm depth of solution in a test tube.
Add no more than 1 cm depth NaOH.
Hold tube at arm’s length, heat gently (do not boil).
Test any gas evolved with damp red litmus paper (turns blue if NH₃ present).
Test D: Carbonate (CO₃²⁻) Test with Dilute HCl
Place 1 cm depth of solution in a test tube.
Add 1 cm depth dilute HCl.
Test any gas evolved with lime water (cloudy if CO₂ present).
Test E: Sulphate (SO₄²⁻) Test with HCl and BaCl₂
Place 1 cm depth of solution in a test tube.
Add 1 cm HCl, observe reaction.
Add BaCl₂ dropwise, shake gently; white precipitate indicates SO₄²⁻.
Test F: Halide Test (Cl⁻, Br⁻, I⁻) with HNO₃ and AgNO₃
Place 1 cm depth of solution in a test tube.
Add 1 cm HNO₃, observe reaction.
Add AgNO₃ dropwise, shake gently; precipitate colour identifies halide:
Cl⁻ → white (AgCl)
Br⁻ → cream (AgBr)
I⁻ → yellow (AgI)
Conclusion: