lecture 4: recent technological advances in EM and their applications to solving macromolecular structure
electron microscopes in the past were kept in dark basements so that vibrations didn’t disturb the images as well lights disturbing the fluorescent screens they were viewed on. Single particle work had been the poor relation of the other techniques due to the low resolution images they produced.
the rolution revolution and direct electron detectors
Cryo-em has some limitations:
poor signal to noise due to having to take pictures with low dose and images formed by phase contrast and not amplitude
slow and laborious and can break expensive machinery
limit on size
although theoretically can go as small as 38 kDa, in practice 300 kDa was the limit
so for small low symmetry proteins, this is hard and produces low resolution although this has improved
In 2013, papers were published were the resolutions was of 3 angstroms for single particles. This was the first wave of the resolution revolution. There were a series of improvements that built up over the years including automated data collection, development of energy filters, improving microscopes as well as better software for reconstructions and direct electron detectors which were new cameras that became available.
The first ways of digitising images came from CCD devices which is a charged couple detector where there are a series of process required to go from the information carried to the actual image. In electron microscopy electrons stimulate photons in a layer that are transported by a fibre optic layer to another layer. This process allowed the image from the electrons to be stored as a data file. Multiple process means that sometimes the signal can be lost. Another chip technology came along that allowed direct electron to electron information passage.
The improvements were compared to film which was continued to be used through the CCD age as it is a chemical change that occurs when electrons it the film and so gave a good range of signal. If you take a lot of pictures on film, they must be developed and you don’t know what they look like until then. CCD is rapid however, they don’t give the depth of information tfilm did. direct doctors gave good contrast and preservation of information.
Direct electron detectors are very sensitive however, they can also produce movies by the nature in which they operate by taking maky images on a rapid timescale. Previously detectors required 1 second exposures to produce a single image and long exposure time leads to loss of resolution due to sample drift. Capturing dynamic process is useful as there are negative dynamic process that occur when you are trying to take pictures and this is because the frozen sample below 190 degrees C should be kept still for the 1 second and if it moves, the image contains lots of information of the movement. Movement distrups final resolution as the final resolution is from many images of the same structure in the same place and so movement limits the resolution to the amount the sample moved. Direct electron detectors allowed the potential to capture movement ahd correct for it by using cross correlation methods to follow the movement.
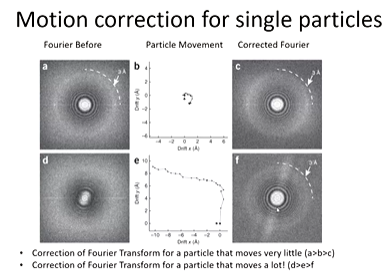
Motion correction corrects for sample motions and sample degradation. wHITE LINES SHOW PARTICLE MOVEMENT AND BLUE IS THE AVERAGED PARTICLE TRACk
Fourier transforms - structural information and high quality means there are clear rings towards the outside. This can be because the particle movement is small. This means that the image looks less blurry
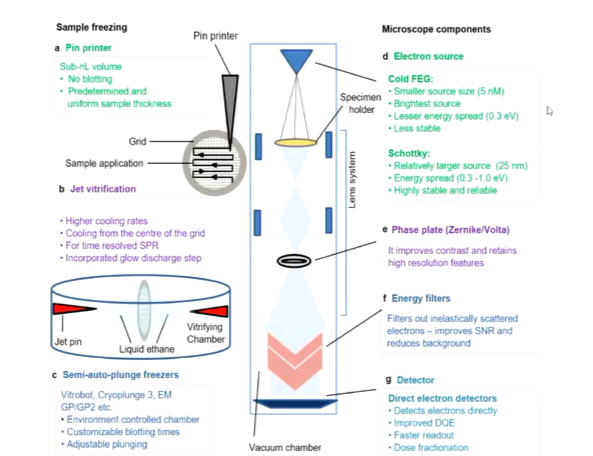
Structural information in images comes from interference between inelastically scattered and unscattered electrons. in inelastically scattering, noise is created and creates noise in the image. These have not lost energy so that means they have similar properties and so if a magnetic lense is applied and divert their path, they should all follow this path. This is what the energy filters do so you reduce the amount of noise to improve contrast.
Energy filters improve contrast to help with image processing
Phase plates introduce phase contrast at focus under conditions to get the best resolution. To get a good image on an electron microscope, you have to focus however then the contrast drops to a minimum. This problem was solved by the phase plate which can change the phase of the electron at the image.

different samples have different optimal conditions for cryopreservation. automated blotting and freezing allows for greater consistency between samples. There has been a huge effort to optimise the way samples are preserved.
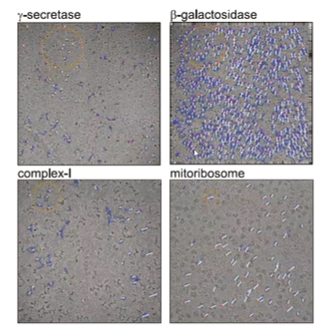
game changing examples
ribosomes
The first 3D structure of ribosomes was obtained by negative stain EM in 1974 and the first 3D crystals were obtained in 1980. Medium resolution cryo-EM were obtained from about 1996 and these were useful for trying to work out conformational changes and the structure. The first X ray structures appeared in 2000 and this showed specific molecules in the structure.
The cryo-em resolution revolution were obtained from 2014 with near atomic resolution. This meant that they could do more structures and now lots of high resolution ribosomes in different conformational stages and bound to their peptides and drugs have been obtained
membrane proteins
membrane proteins are often difficult to crystallize. gamma-secretase is an enzyme that produce ab-peptides which is linked to Alzheimer’s. 3.4 angstrom resolution images were obtained ad showed mutations associated with increased risk in Alzheimer’s.
continues milestones
there was an outbreak of zika virus that was new to a larger population that can cause paralysis and neurological defects in embryos. the structural biology was unknown but quickly a 3.5 angstrom structure was released and this was also the same with covid.
Another milestone was the improvement in resolution which got down to 2.2 angstrom using new methods. the resolution revolution allowed higher resolutions of proteins and complexes to be obtained.
cryo electron tomography
cryo-electron tomography gives 3D structures of virtually any biological specimen. In this way, one structure that could be complicated and obtain a 3D structure for that. There is no need for averaging procedures like in single particle analysis. This therefore bridges the gap between light microscopy and protein structure determination methods.
tomography works by the sample in the microscope having its picture taken - a 2D projection. MOre pictures are taken in different angles and orientations of the sample. These 2D projections are collected and as the orientations are knon, back projection can be done to construct the 3D structure. However, this can damage the sample over time as electrons are in the beam. This reduces the resolution.
Other limitations mean that the sample has to be thin 5000 nm thick. This can be okay for prokaryotic cell. Electron dose needs to be between 3000-5000 electrons nm-2.
further information can be obtained by correlative light and electron microscopy and this puts higher resolution in a cellular and historical context. the process of mapping two different scales is very hard. cells are grown on grids and frozen and have tomography carried out on them. the result is that we know which cellular structure accommodates a synaptic vesicle for example.
high resolution 3D cell biology
recent advances have implications for tomography too. kt was realised that averaging can be done with tomograms as there can be features that are identical in structures. This means that high resolution can be done by averaging in tomography.