Chemistry Unit 2
Atomic Models:
Democritus - first atomic model. discovered atoms
John Dalton - created atomic theory. his model was the same as Democritus’s
Dalton’s atomic theory: 5 components -
Matter is made up of atoms, small and indivisible particles.
All atoms of the same element are identical and have the same mass.
Atoms of different elements vary in size, mass, and chemical behavior.
Chemical compounds are made up of at least 2 atoms of different elements. The resulting particle is called a molecule.
In a chemical reaction, atoms are rearranged, separated, or recombined to form new compounds but no atom is created or destroyed.
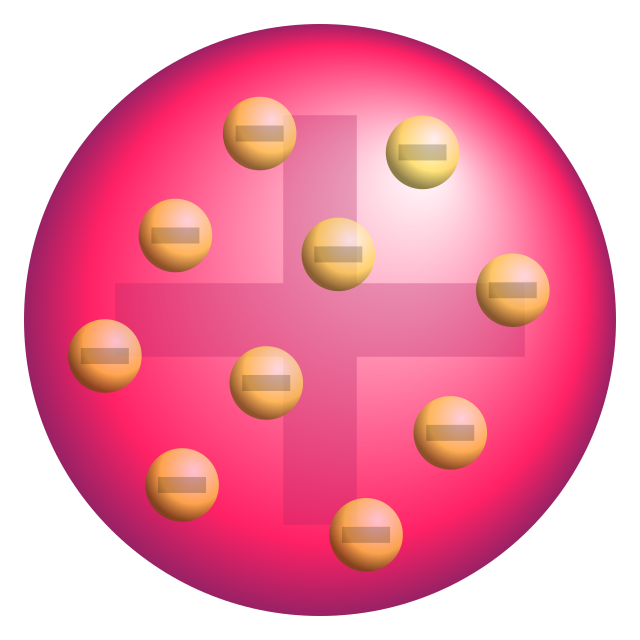
JJ Thompson - discovered the electron using cathode ray tube experiment. choc chip model
Ernest Rutherford - discovered the nucleus + atom is mostly empty space using gold foil and ray experiment. created the nuclear model
Niels Bohr - discovered that electrons move in orbits around the nucleus. though they were definite. created the planetary model

Erwin Schrodinger - discovered that electrons move in clouds not definite orbits. created quantum model

Chemistry Unit 2
History of the Atom
Democritus - first atomic theory
Dalton - Atomic theory
JJ Thompson - Cathode ray tube experiment -> discovered electron ->choc chip cookie model
Ernest Rutherford - gold foil experiment -> discovered positively charged nucleus + atom is mostly empty space -> nuclear model
Bohr- discovered electrons orbit around nucleus->planetary model
Schrodinger - discovered electron clouds -> quantum model
Atoms, Ions, and Isotopes:
Atoms
Periodic Table: Atomic number is on top, atomic mass is on bottom. Mass is almost always larger. Hydrogen is the same because there are no neutrons.
Protons = atomic number, Electrons = protons, neutrons = mass-atomic number
Ions
Atoms have neutral charge while ions have lost or gained electrons
Cation + or anion -
If positive, then electrons have been removed
If negative, then electrons have been added
Isotopes
Number of neutrons differ, so atomic weight changes
Standard nuclear notation: numbers are to the left. Mass number is on top, atomic number is on bottom. To the right is the element. Just look at which number is bigger to figure out which is the mass.
Hyphen notation is the element - mass number. The atomic number will not be added because the atomic number never changes!
Light
Light can be shown as a wave or stream of photons
Frequency = how short or long the wavelength is. Shorter wavelength means higher frequency.
Wavelength is the distance from crest to crest. Crest is the top of a wave, trough is the bottom of the wave.
Electromagnetic spectrum
From lowest to highest frequency/energy.
Radio, Microwave, Infrared, Visible (ROYGBIV), Ultraviolet, X-ray, Gamma ray.
Photoelectric effect: when light hits a metal, electrons are released (light above a certain frequency)
2 ways to increase it are to increase the frequency or to increase the intensity
Light and electrons:
When an atom absorbs energy/light/photons, its electrons move away from the nucleus. Ground state -> excited-> ground. Release a photon when it goes back to ground.
Energy is proportional to what it absorbs. If it absorbs violet light(wave length), then it will emit violet light.
Light equations
e=energy = hv. Energy is measured in joules
h=plancks constant
v=frequency. Units are s^-1 or Hz
v=speed of light over wavelength in meters (c over upside-down y (its a greek letter))
Atomic Spectra
The unique range of wavelengths emitted by an atom/element. Unique for every element
Each atom can be identified by the light it emits
Electron Configuration
The arrangement of electrons in an atom
Number = energy level/quantum number
Letter is the sublevel or shape of orbital
^number is the number of electrons in that sublevel
Periodic table shows sublevels
Orbitals:
Two arrows to show how many electrons occupy it. Opposite spins = opposite arrows.
Exceptions: some elements go from 4s^2 3d^4 to 4s^1 3d^5 because half filled orbitals are more stable than a full and 4/10ths full orbital. This ex was chromium, but copper, silver and molybdenum (cr, cu, ag, mo) are all exceptions to know.
Noble gas configuration is the same as electron configuration but instead it starts with the noble gas above and continues until the element.