Chemistry
Protons - a stable subatomic particle in the atomic nuclei w/ a positive charge equal in magnitude to that of an electron, but of opposite sign
Neutrons - A subatomic particle of about the same mass as a proton but w/out an electrical charge
Electrons - A negative charged subatomic particle that is 1/1836 times smaller than a proton
Electrons constantly move around the nucleus of an atom made up of protons and neutrons
The number of electrons and protons are equal
Atomic # = # of protons in an element
Atomic mass = home many protons and neutrons are in an element
How to find neutron # → neutrons = mass # - atomic #
Mass of 1 proton: 1.67×1024 or 1 amu
Mass of 1 neutron: 1.67 × 1024 or 1 amu
Mass of 1 electron: 9.109 × 10-25 or .00059 amu
Electrons are not considered in the mass # of an atom
Isotopes - Distinct nuclear species of the same chemical element. They have the same # and position in the periodic table, but differ in the # of neutrons
Same element but w/ different weights
Ions - An atom or group of atoms w/ a net electrical charge.
Atoms becomes an ion if it gains or loses electrons
Cation - positively charged atom → atom loses electron
Anion - negatively charged atom → atom gains an electron
“Cation are ‘paw’sitively charged”
Shell - electron revolve around the nucleus in a specific circular path known as orbit or shell
Subshell - A division of electron shells separated by electron orbital
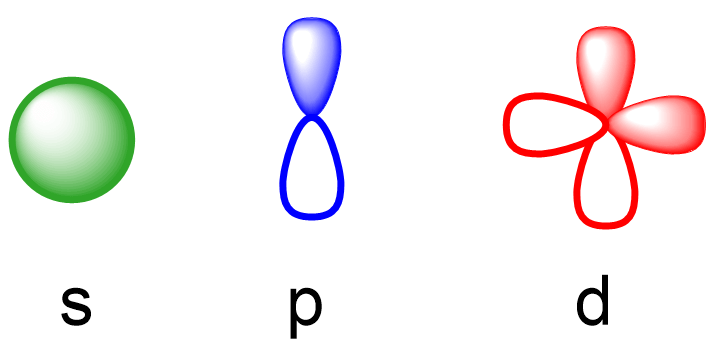
Every shell has a subshell of s,p,d,f
Only electrons in the outermost shell participate in chemical bonds
| N=1 | K shell | s |
| N=2 | L shell | s,p |
| N=3 | M shell | s,p,d |
| N=4 | N Shell | s,p,d,f |Orbital - 3 dimensional space within an atom where an electron in a given subshell can be found
S orbital
P orbital
D orbital
Shell #1:
Subshell: S
# of orbitals: 1
Max # of electrons: 2
Shell #2:
Subshell S,P
# of orbitals: 1,3
Max # of electrons: 8
Shell #3:
Subshell S,P,D
# of orbitals: 1,3,5
Max # of electrons: 18
Shell #4:
Subshell S,P,D,F
# of orbitals: 1,3,5,7
max # of electrons: 32
# of orbitals increase by +2. Electron # increase by 4 per subshell
EX: If magnesium has an atomic # of 12, it should be written out like:
1s²2s²2p63s2
w/ the subscripts adding up to 12
Ionic bonds- Complete transfer of valence electron(s) between atoms
Octet rule - Atoms prefer to have 8 electrons in their valence shell. They may lose, gain, or share electrons
Ionic bonds have an “I take, you give” relationship
EX: NaCl
Covalent bond - Sharing of electron pairs between atoms
Typical between 4 major elements:
Carbon
Oxygen
Hydrogen
Nitrogen
“CHON”
Periodic Table
Row = Period
Indicates how many electron shells an element has
Columns = Groups
Indicates the # of valence electrons the element in the group has
Doesn’t include transition metals (elements in the middle)
Physical Properties and Changes of Matter
Matter - Anything that has weight and occupies space/volume. Can be solid, liquid, or gas
Mass - The amount of matter an object contains
Can be measured in grams (g) or kilograms (kg)
Volume - The amount of space an object occupies
Can be measured in liters (L) or milliliters (mL)
Density - The relationship between the mass and volume of an object
To calculate: Density = mass/volume
Solid:
has definite shape
Has definite volume
Can’t compress
Liquid:
Has indefinite shape (takes shape of container)
has definite volume
Can’t compress
Gases
Has indefinite shape (takes shake of container)
Indefinite volume (changes as it expands and contracts)
Compressible
Temperature causes particles to increase its vibration
Increased pressure + lower temperatures can cause gasses to transition into liquid
Melting - When a solid gains heat and changes into a liquid
EX: Ice → Water
Freezing - Process where a liquid loses heat and turns into solid
Water → Ice
Condensation - Process where a gas loses heat and turns into a liquid
Clouds created when water vapor in the air cools
Evaporation - Transformation of a liquid into a gas
Sublimation - Process where a solid changes directly into a gas w/out first becoming a liquid
Deposition - Also known as desublimation. When a gas changes directly into a solid w/out first becoming a liquid
EX: Frost forming on a window
Chemical Reaction
Chemical Reactions - One or more substances, known as reactants, that are transformed into different substances called products
Reactants - substances that present at the start of a chemical reaction that participates in the reaction
Products - Substances that are formed as a result of a chemical reaction
Reactants are the ingredients to make the products
Combination - Two or more substances combine to form a single product
EX: A + B → AB
Decomposition - A single substances breaks down into two or more substances
EX: AB → A + B
Single Displacement - One element in a compound is replaced by another element
EX: A + BC → B + AC
Double Displacement - Elements in two different compounds swap places w/ each other to form new compounds
EX: AB + CD → AD + BC
The inner elements combine and the outer elements combine to form new compounds
Combustion - A substance (usually a hydrocarbon) reacts w/ oxygen to produce heat, light, and typically produces carbon dioxide and water
EX: CxHy + CO2 → CO2 + H2O + Fire
Hallmark sign of combustion: The second reactant is oxygen and the products are carbon dioxide and water
Balancing chemical reactions - Reactants are the same exact # as your products
Save oxygen & hydrogen balancing for last
Moles
Moles - Units of measurement that is the amount of pure substance containing the same # of chemical units
Mole = 6.022 × 1023 → Avogadro’s #
To calculate the molar mass, multiply the amount of elements by the element’s mass and add them all together
EX: C6H8O6 = (6×12) + (8×1) + (6 × 16) = 176 g/mol
Factors that Affect Chemical Reactions
Collision Theory - for particles to react, they have to collide w/ each other w/ sufficient energy (activation energy)
Amount of energy of particles:
More energy → more energy they transfer when they collide
less energy → nothing will happen, unable to reach activation energy
Temperature:
higher rate of reactions
Particles gain more energy → particles move faster & collide harder/more often → more likely to exceed activation energy
Concentration/pressure:
How many particles per unit of volume
More particles per unit of volume → collide more frequently → higher rate of successful collisions
Surface area:
More surface area facilitates more collisions between atoms → higher rate of successful collisions
Catalyst - Higher rate of reaction w/ less energy
Substances that speed up a reaction w/out being used up in the reactions themselves
An alternate reaction pathway
Exothermic reactions - energy/heat is released at the reaction
Combustion reactions
Oxidation reactions
Neutralization
Endothermic reactions - Takes in heat energy from the surroundings. Absorbs heat
Photosynthesis
Cooking an egg
Liquid evaporation
Equilibrium - When the rate of the forward reaction equals the rate of the reverse reaction in a closed system
Reactant 1 + reactant 2 → product
Reactant 1 + reactant 2 ← product
Static equilibrium - The forward and reverse reaction are occuring at the same time
Continuously happening
Properties of Solutions
Polarity of Water
Adhesion - Binding or attraction between dissimilar molecules, atoms, surfaces, and substances
Cohesion - Attraction of molecules for other molecules of the same kind
Solution - A homogeneous mixture of one or more solutes dissolved in a solvent
Homogeneous mixture - when the solutes, such as salt, is completely dissolved in the solvent, such as water. Not easily separated
Heterogeneous mixture - the solute and solvents are still easily separated such as pebbles in water
Solutes - A substance that can be dissolved into a solution by a solvent and is present in smaller amounts
Solvent - A substance with the ability to dissolve other substances to form a solution and is present in larger amounts
Water is called the universal solvent because it is capable of dissolving more substances than any other liquid
Polar substances - soluble in water
Non-polar substances - not soluble in water
Molarity - # of moles of solutes per one liter of solution
M = moles/liter
Dilution - process of reducing the concentration of a solute in a solution by adding more solvent
Hypertonic - has a higher solute concentration. Solutes flow out
Hypotonic - has a lower solute concentration. Solutes flow in
Isotonic - contains the same concentration of water and solutes
No osmotic flow
Osmosis - diffusion of solvent molecules (water) through a selectively permeable membrane from a region of high water potential to a region of lower water potential
Factors affecting diffusion:
Distance - the greater the distance, the slower the diffusion rate
Temperature - Higher temperature causes an increase in diffusion rate
Solvent characteristics - Increased density can slow molecules down, decreasing diffusion
Traveling characteristics - The greater the mass, the lower the diffusion rate
Barrier characteristics - small non-polar cells pass through the barriers more easily
Acid and Bases
A measure of how acidic or alkaline a solution is
Acid - substances that increase concentration of hydrogen ions (H+)
Base - substances that decrease concentration of hydrogen ions (H+)
Measuring pH:
Wide range indicator - chemical compound that changes color based on pH
Color corresponds to pH level such as red for acidic or blue for alkaline
Universal indicator - color range from deep red in very acidic to blue/purple to very alkaline
Type of wide range indicator
Litmus paper
Blue litmus paper turn red under acidic conditions
Red litmus paper turn blue under alkaline conditions
pH probe - gives more accurate readings of pH
When acids and bases are mixed, they undergo neutralization
Typically results in salt or water
o
Acids often tend in -ic such as hydrochloric acid
Bases often end in -oxide or -nate such as sodium hydroxide or calcium carbonate