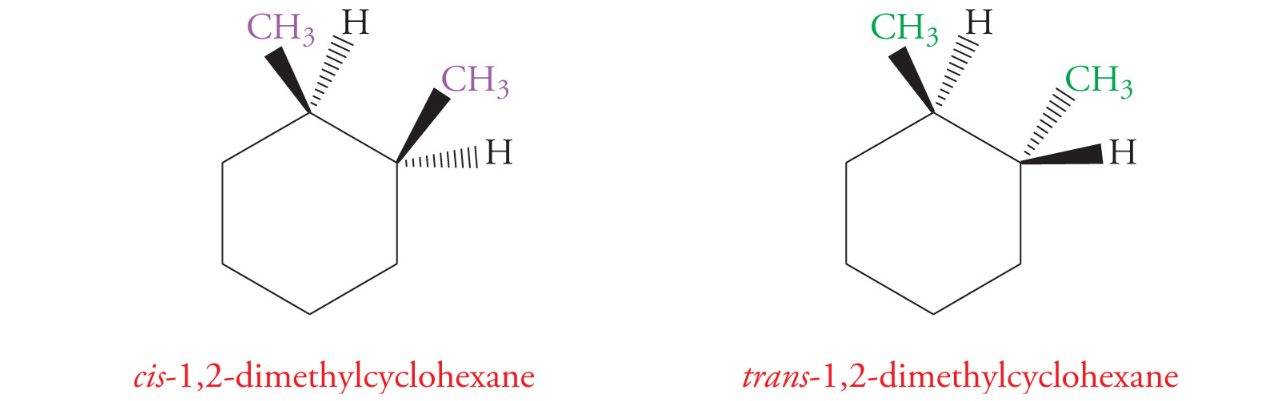4.2 Isomerism
Summary of Isomerism
Constitutional Isomerism
Constitutional (structural) isomers: compounds that have the same molecular formula but have their atoms connected together differently.
EXAMPLES:
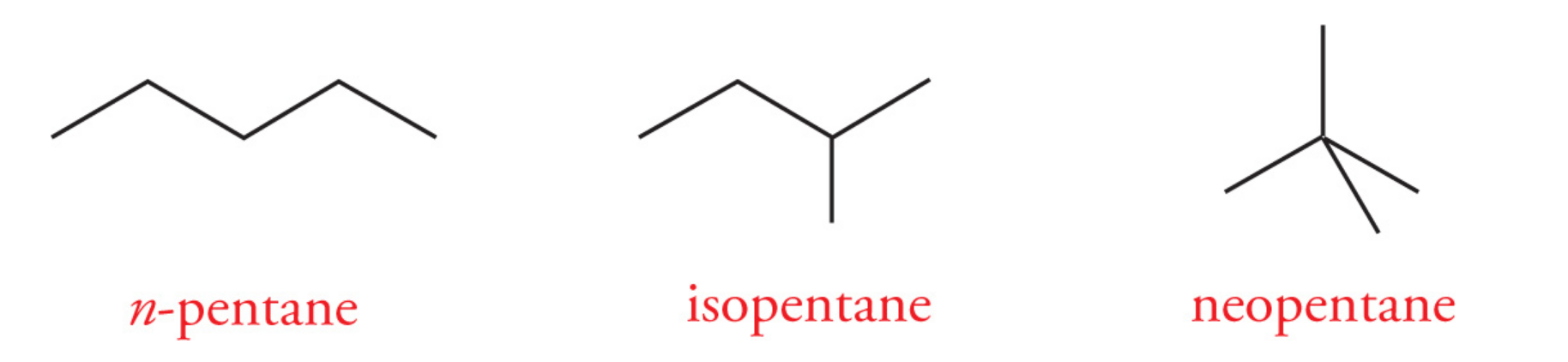
pentane (C5H12) has two additional constitutional isomers:
Isopentane and Neopentane
n-pentane (the n- prefix means normal, when carbons are unbranched and continuous)
Hexane (C6H14) has five total constitutional isomers:
n-hexane
2-methylpentane
3-methylpentane
2,3-dimethylbutane
2,2-dimethylbutane
Conformational Isomerism
Conformational isomers are compounds that have the same molecular formula and the same atomic connectivity, but differ by rotational about a sigma bond
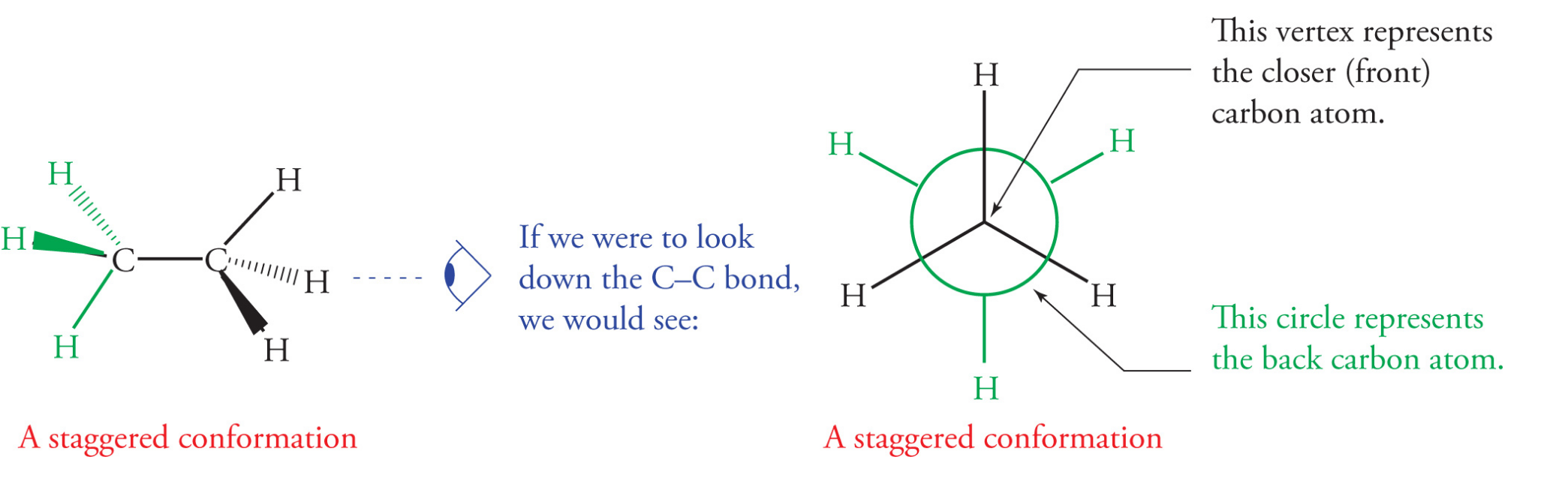
For saturated hydrocarbons, there are two orientations of sigma bonds attached to adjacent sp3 hybridized carbons; staggered and eclipsed
Staggered conformation: a sigma bond on one carbon bisects the angle formed by two sigma bonds on the adjacent carbon
less crowded, more stable
2 types of staggered conformations:
Gauche: when the two largest groups on adjacent carbons are in a staggered conformation 60 degrees apart (contains relative minimum amount of energy)
higher in energy than anti conformations due to steric strain (repulsion between electrons between the two larger groups)
can be more stable if groups are not too large and can form intramolecular hydrogen bonds with one another
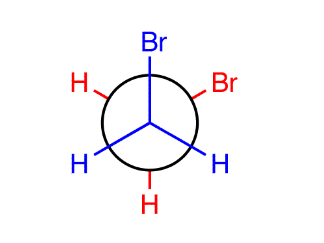
Anti: when two largest groups on adjacent carbons are in a staggered confirmation 180 degrees, has an absolute minimum of potential energy, usually more stable (if two groups are too large)
Eclipsed conformation: a sigma bond on one carbon directly lines up with a sigma bond on an adjacent carbon
more crowded, electron repulsion, less stable
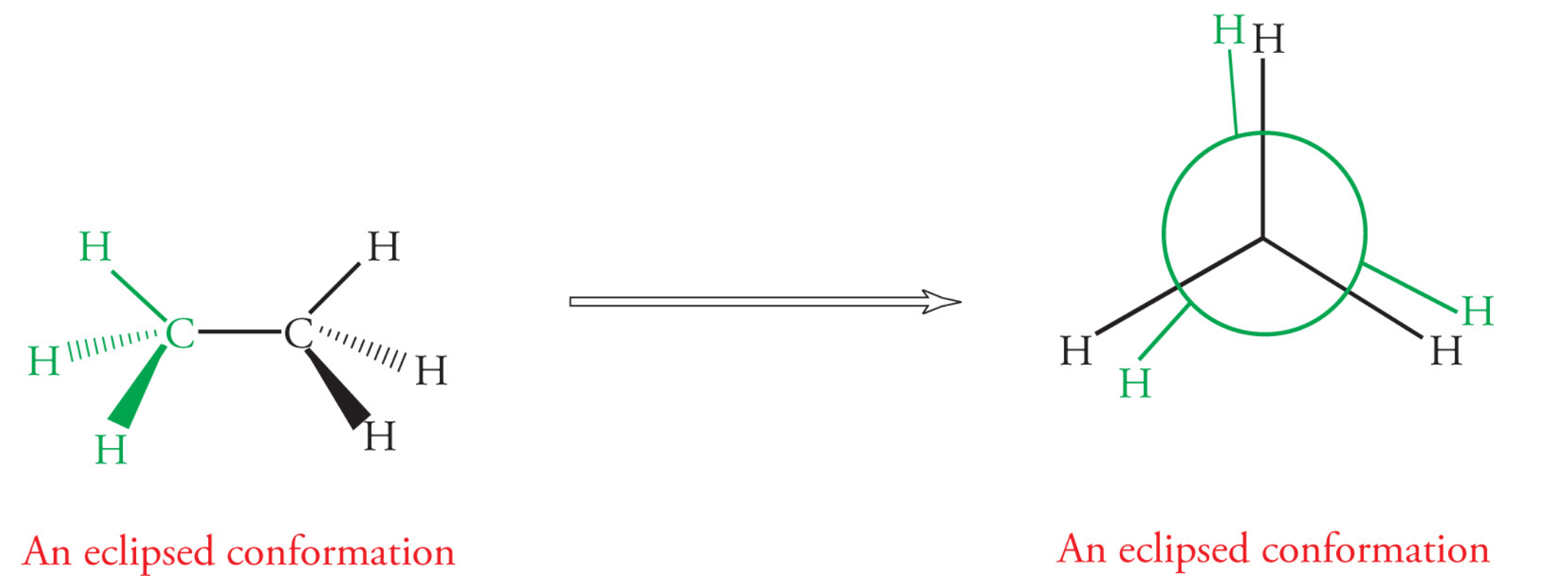
Eclipsed conformations with large molecules beside each other have the highest amount of energy (absolute max) due to steric strain (syn conformation)
Eclipsed conformations with two largest groups not ontop of each other have a relative maximum amount of energy
Cyclohexane/Cyclopentane conformations
Cyclopentane has a pentagonal bond of 108 (which is close to the tetrahedral 109 degrees).
The cyclopentane takes up a puckered envelope form in order to prevent carbon-hydrogen bonds from eclipsing each other.
Energy of the compound is reduced due to staggering present
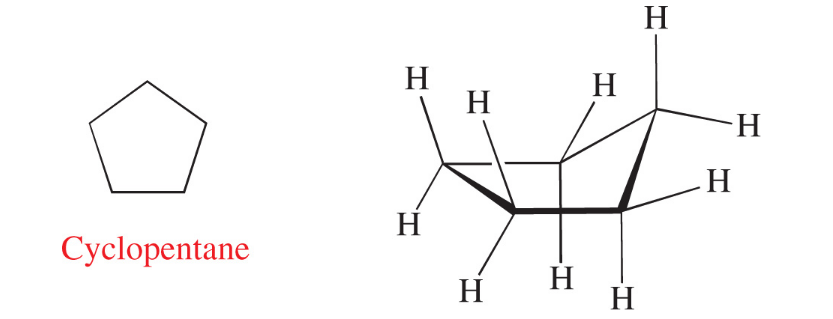
If cyclohexane was planar, they would have bond angles of 120 degrees.
being in planar structure would cause considerable strain on sp3 hybridized carbons since the ideal bond angle should be around 109 degrees
the most stable conformation of cyclohexane is a puckered molecule referred to as the chair conformation. there are two chair conformations for cyclohexane, and they easily interconvert at room temperature.
in order to change between chair conformations, the molecule must pass through several other less stable conformations (half chair conformations that reside at energy maxima) (twist boat conformations at a local energy minimum, but higher energy than chair conformation)
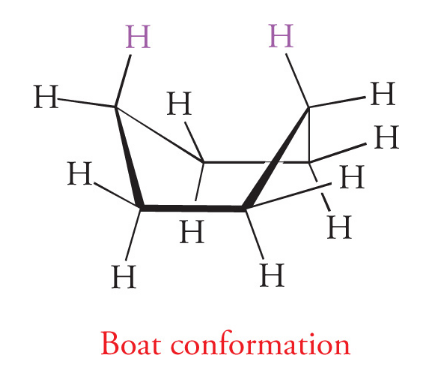
The boat conformation represents a transition state between twist boat conformations, and is much more unstable than chair conformations, thus do not play an important role in cyclohexane chemistry
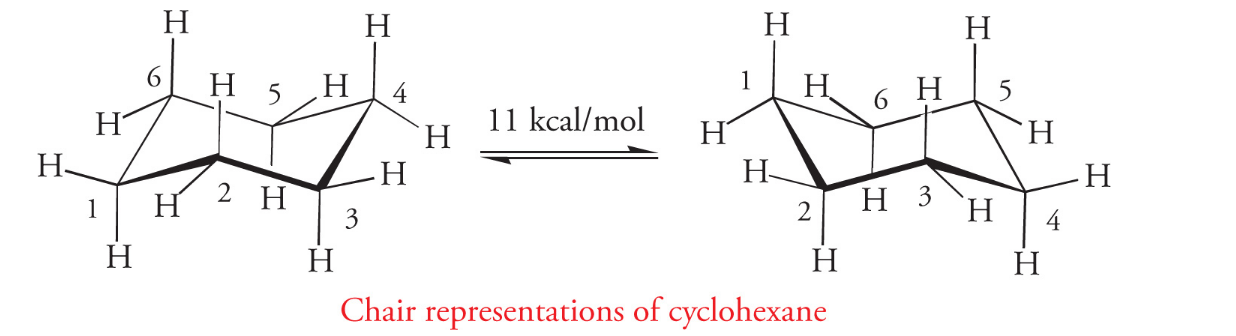
Chair conformations differ by what is on the axial and equatorial planes
what is on an axial of one conformation becomes equatorial in the other conformation
The most stable chair conformation is where large groups occupy the equatorial positions
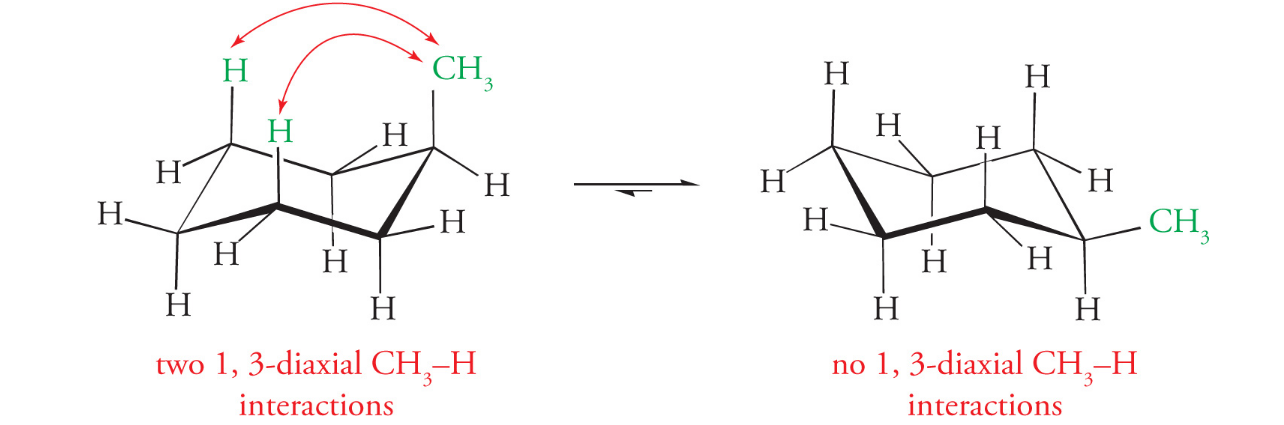
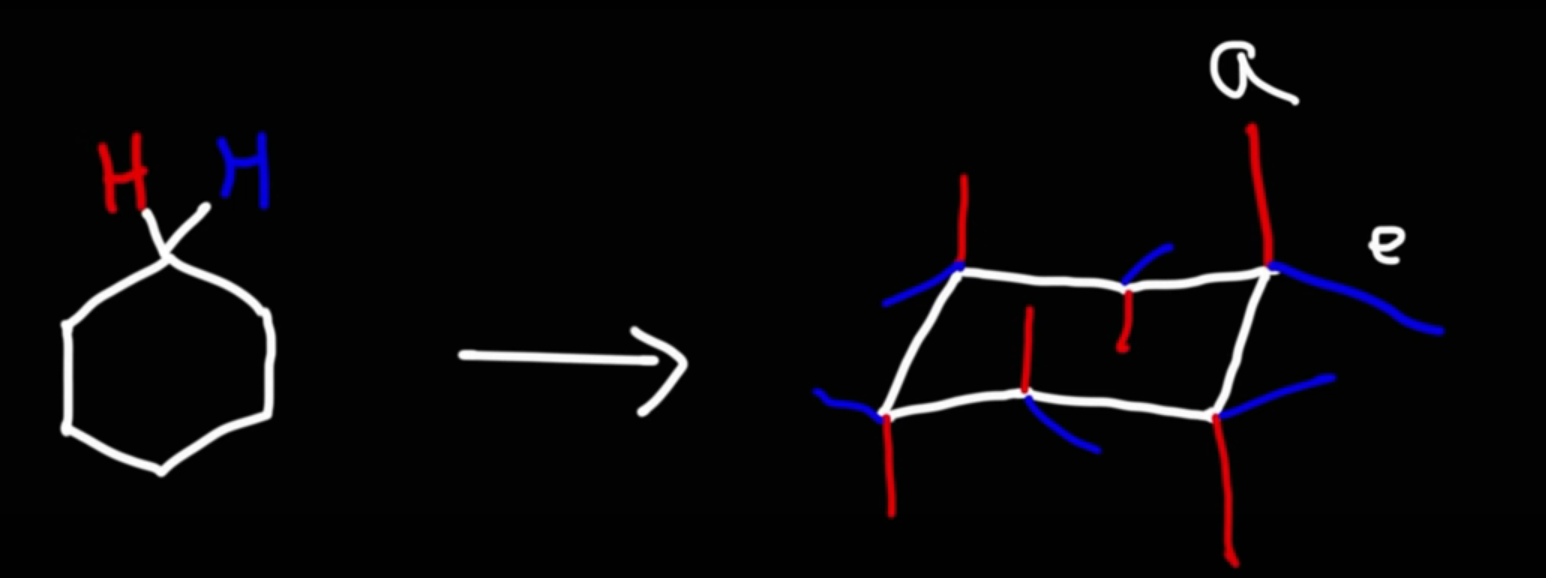
Chair conformation crash course
Axial bond are either straight up or straight down
Equatorial bonds are at an angle, veered off to the side (slants up and then down)
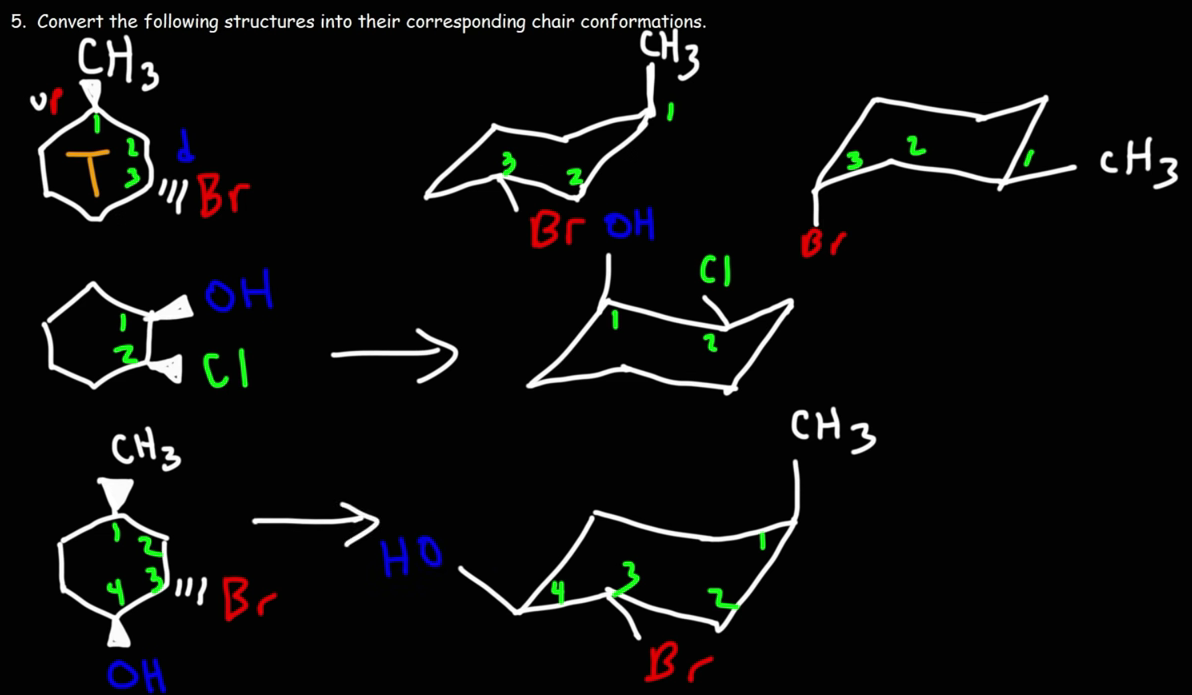
Wedges and Dashes
Wedges go up and dashes go down
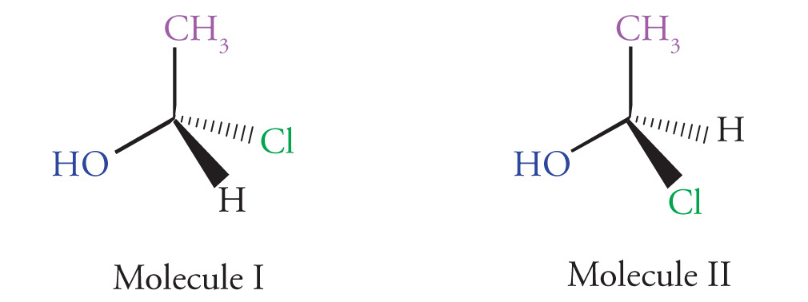
Stereoisomerism
Stereoisomers are molecules that have the same molecular formula and connectivity but differ from one another only in the spatial arrangement of atoms
Chirality
Chiral: any molecule that cannot be superimposed on its mirror image
Achiral: a molecule that can be superimposed on its mirror image and has a plane of symmetry
Chiral centers: have four different groups bonded to it, must be sp3 hybridized with (appox.) 109 bond angles and tetrahedral geometry, also referred to as a stereocenter, stereogenic center, or asymmetric center
Number of possible stereoisomers equals 2^n (where n=number of chiral centers)
Absolute Configuration
Chiral centers can be assigned an absolute configuration.
There are an arbitrary set of rules for assigning absolute configurations to a stereocenter:
Priority assigned to the four different substituents on the chiral center according to increasing atomic number of the atoms directly attached to the chiral center
if isotopes are present, then priority among these are assigned on the basis of atomic weight with the higher priority of being assigned to heavier isotopes
if two identical atoms are attached to a stereocenter, then the next atoms in both chains are examined until a difference is found, once again this is done by atomic number
a multiple bond is counted as two single bonds for both of the atoms involved
example: a double bonded oxygen will count as 2 oxygens instead of 1
Once priorities have been assigned, the molecule is rotated so that the lowest priority group points directly away from the viewer, then simple trace a path from the highest priority group to the lowest priority group
Clockwise direction =R configuration(the right way)
Counter-clockwise direction= S configuration (the silly way)
If lowest priority group is coming towards the page (is on a wedge) flip the answer
if the answer is originally R but the last priority is on a wedge- the actual answer is S
Enantiomers
Enantiomers occur when chiral centers are present and are non-superimposable mirror images
They will always have opposite absolute configurations
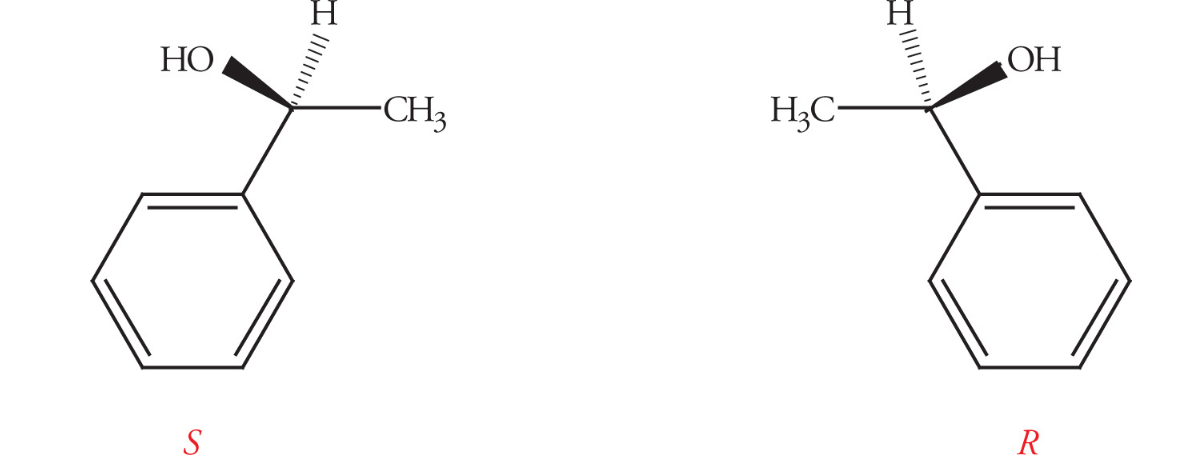
Most chemical properties such as melting point, boiling point, polarity, and solubility are the same for enantiomers but differ in optical activity
Optical Activity
Optically active compounds rotates the plane plane of polarized light
dextrorotatory (d),(+): a compound that rotates clockwise in polarized light
levorotatory (l),(-): a compound that rotates counterclockwise in polarized light
A pair of enantiomers will rotate plane-polarized light with equal magnitude but in opposite directions
when a 50/50 mixture of enantiomers rotate in the same magnitude and in opposite directions, the mixture is racemic and is not optically active (the degrees cancel out to yield the degree of rotation to be 0)
ABSOLUTE CONFIGURATION AND ROTATION SIGNS ARE NOT CORRELATED
Resolution of Enantiomers
A racemic mixture can be separated through a process called resolution
an enantiomerically pure chiral probe, or resolving agent is used to separate the racemic mixture
this is due to interactions of covalent bonds or intermolecular forces (H bonds and salt interactions)
the products yield separated mixtures (that are turned into diastereomers salt) + chiral probes
filtration can then be used to separate the salts and turn them back into enantiomerically pure material
Diastereomers
Diastereomers are stereoisomers that are non-superimposable, non-mirror images… aka not enantiomers
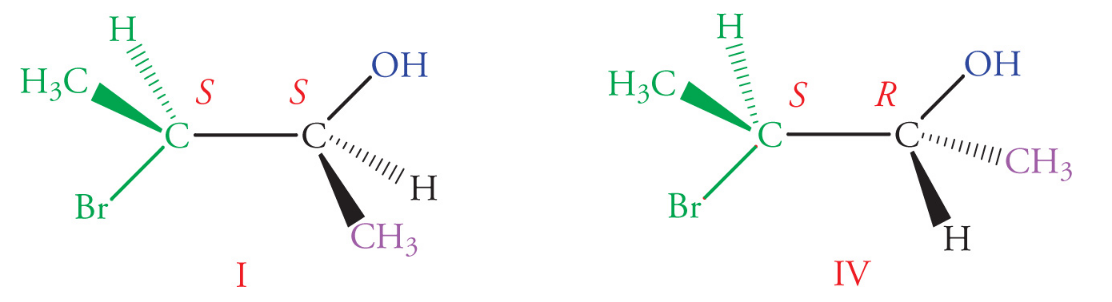
Epimers
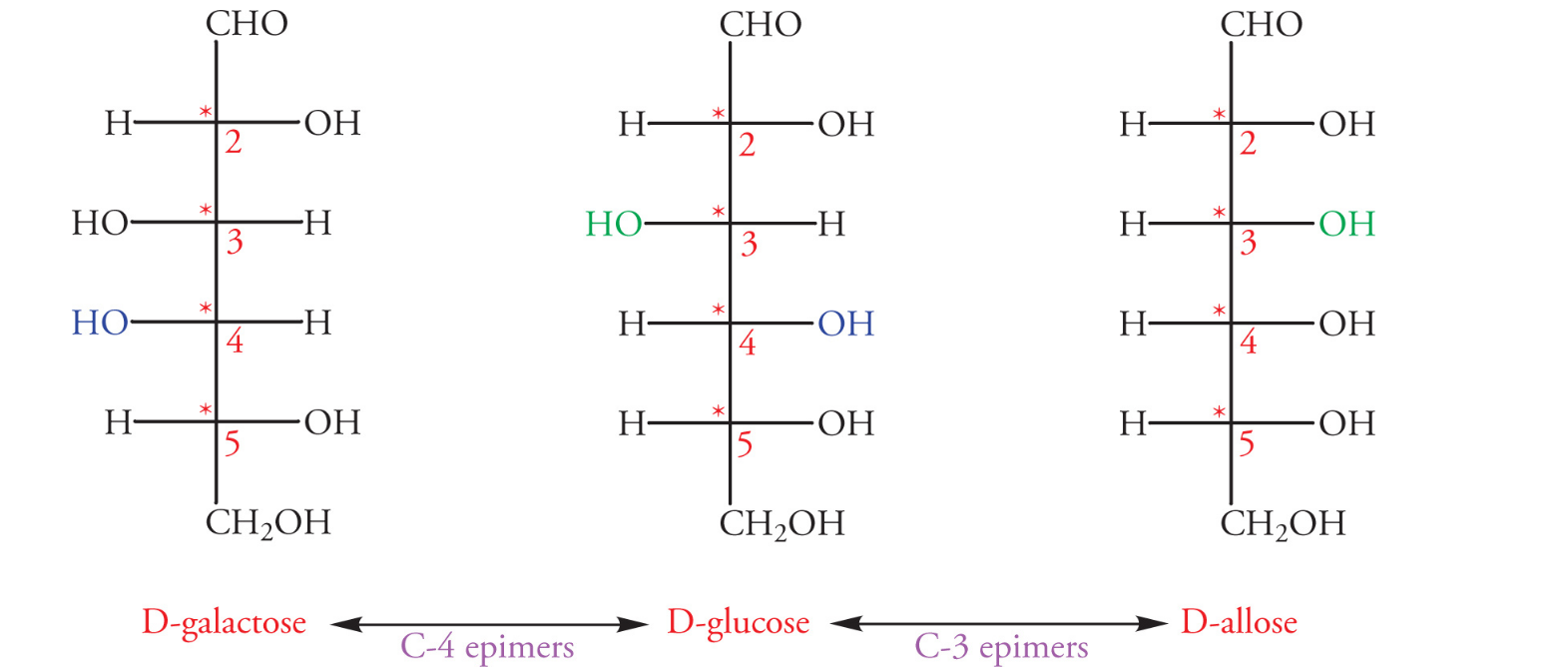
Epimers are a subclass of diastereomers that differ in their absolute configuration at a single chiral center (only one stereocenter is inverted)
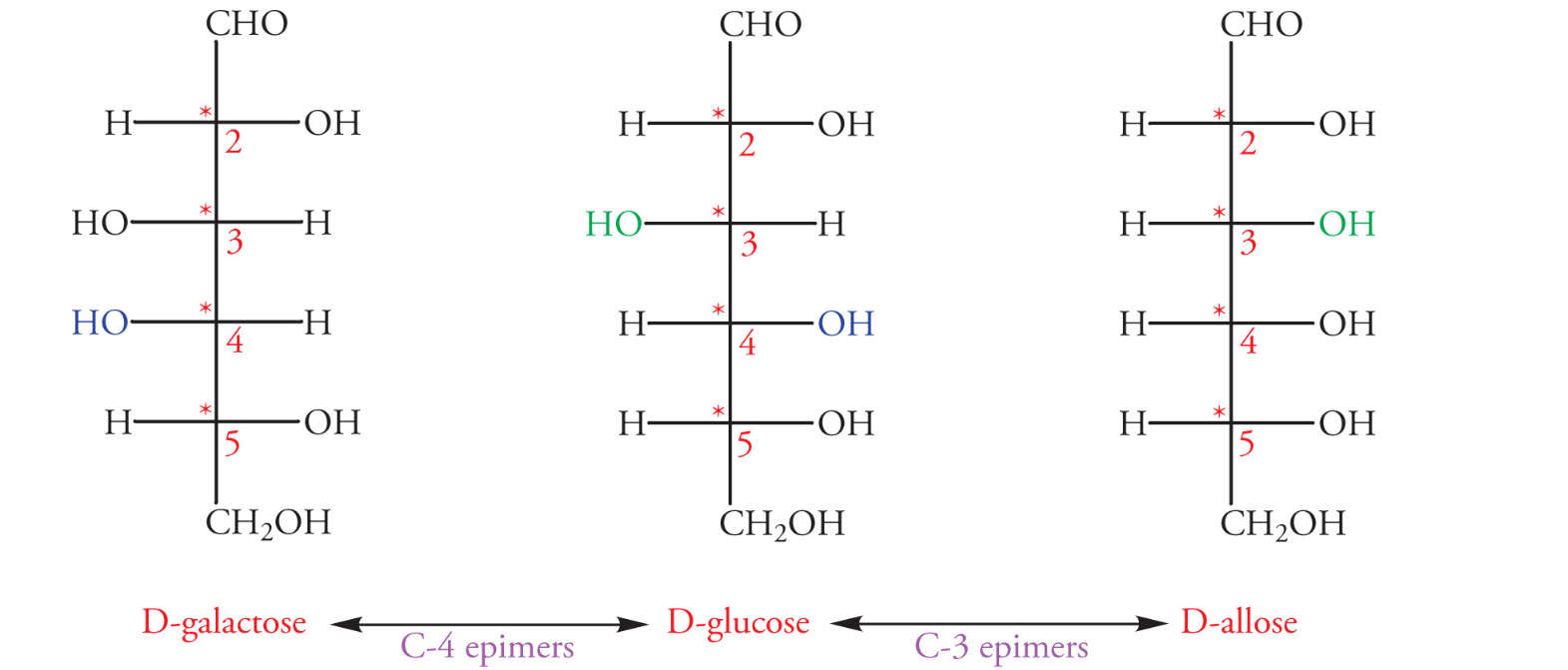
D and L configurations of sugars
D configuration: when the hydroxyl group on the highest numbered chiral center is on the right
L configuration: when the hydroxyl group on the highest number chiral center is on the left
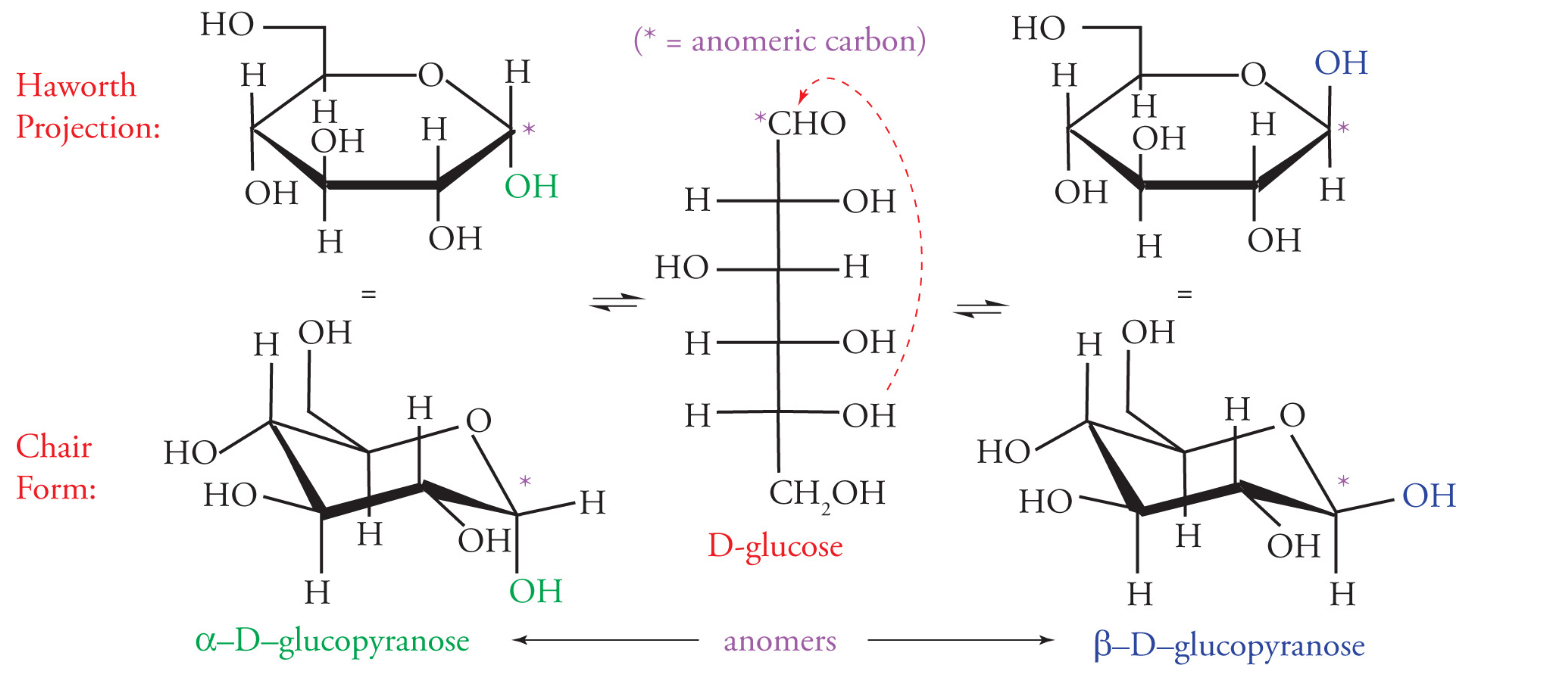
Anomers
Anomers are epimers that form as a result of a ring closure
For the MCAT, anomers will be encountered only with regards to sugar chemistry
A new stereocenter is formed at C1 (anomeric center/carbon) and can result in 2 forms:
Beta: the hydroxyl group is up
Alpha: the hydroxyl group is down
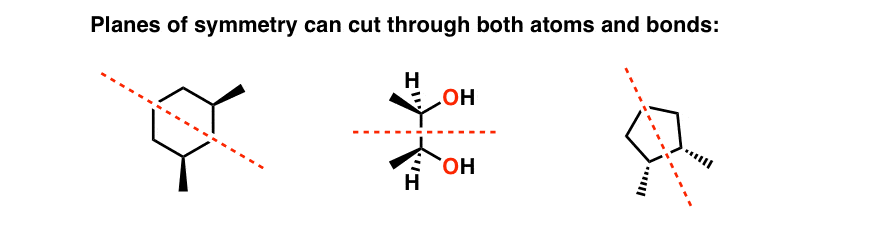
Meso Compounds
Meso compounds are chiral compounds that contain an internal plane of symmetry
they are NOT optically active
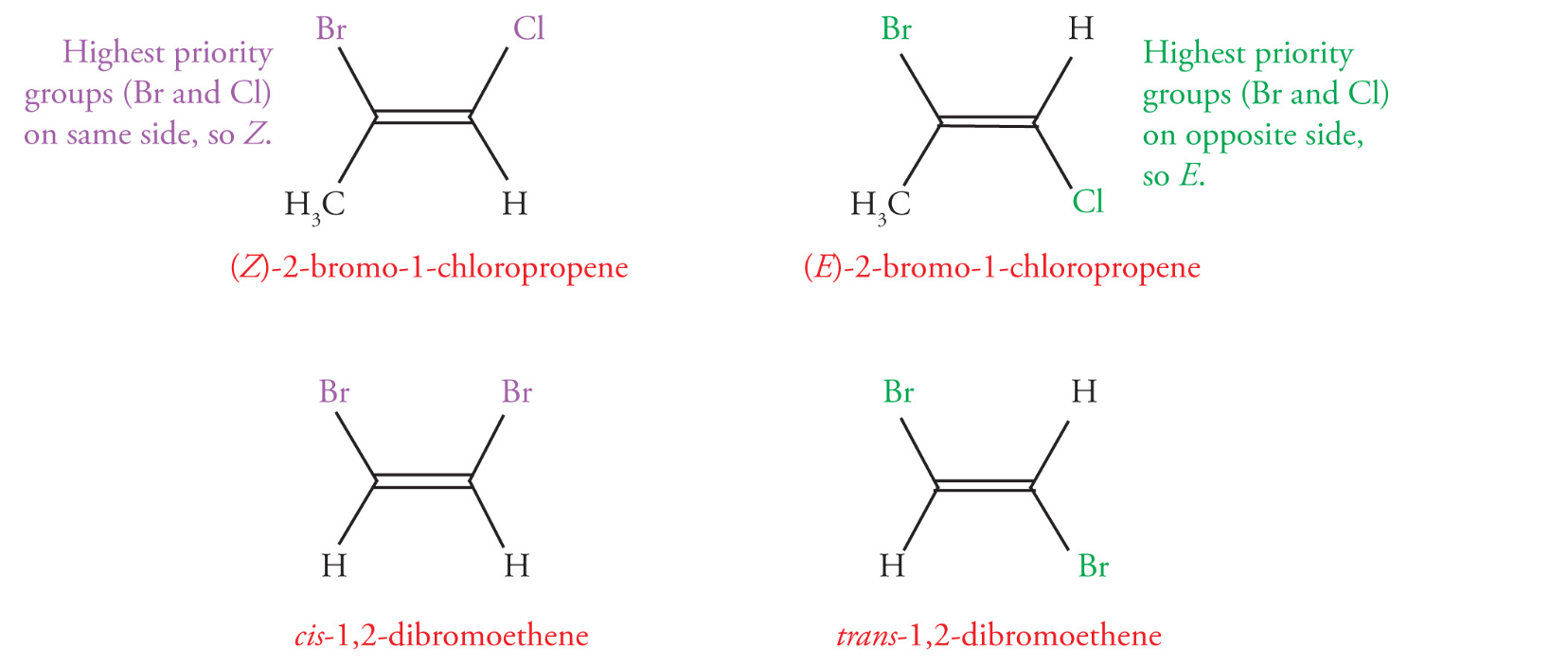
Geometric Isomers
Geometric isomers are diastereomers that differ in orientation of substituents around a ring or double bond
cis/Z- when higher priority groups are on the same sides
trans/E- higher priority groups are on different sides