Chemical bonding structure and properties
Key definitions:
Ion: charged species that are formed by the loss or gain of electrons
Compound: Species made up of more than 1 type of atom
Ionic compound: Compound formed by the electrostatic attraction of oppositely charged ions
Brittle: Something hard that will snap when force is applied
Intermolecular forces: Forced acting between molecules
Delocalised: not in a fixed position
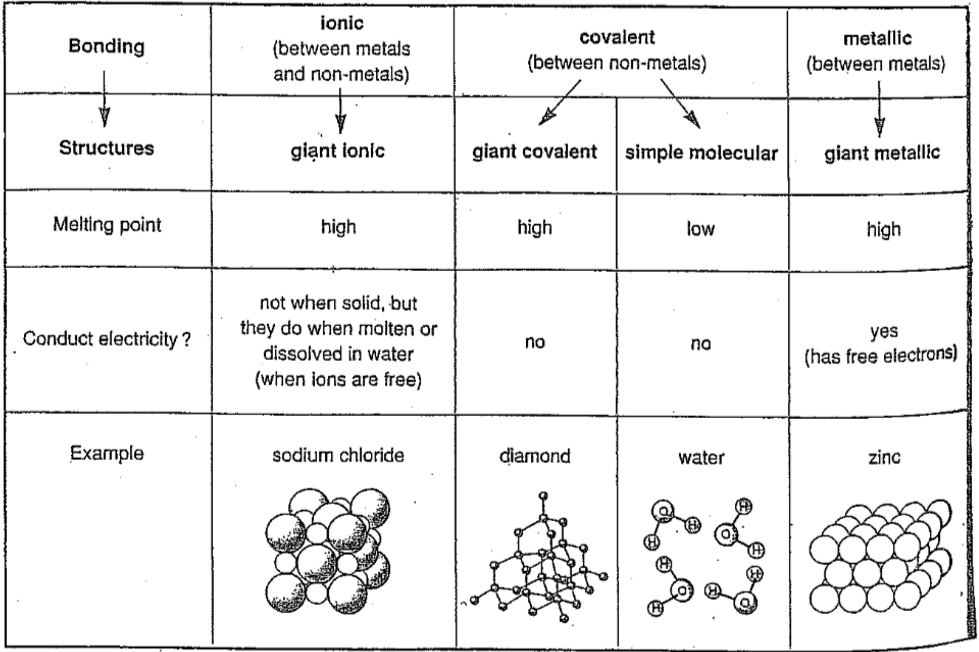
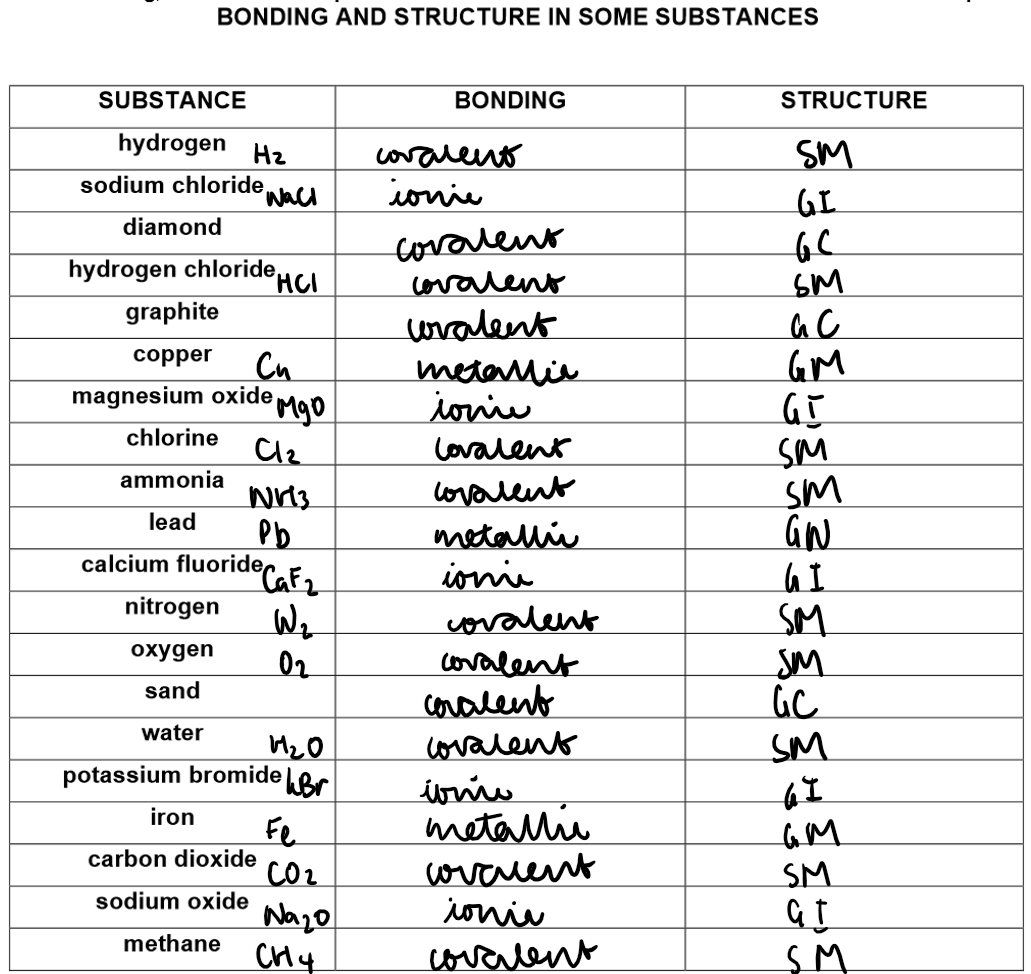
Ionic Bonding:
Formed by electron transfer from a metal to a non-metal.
Creates oppositely charged ions.
Ions are charged atoms or groups of atoms formed by electron loss or gain.
Metals lose electrons (MALE: Metals Are Losers of Electrons) → form positive ions (cations).
Non-metals gain electrons → form negative ions (anions).
Bonding involves strong electrostatic attraction between the oppositely charged ions.
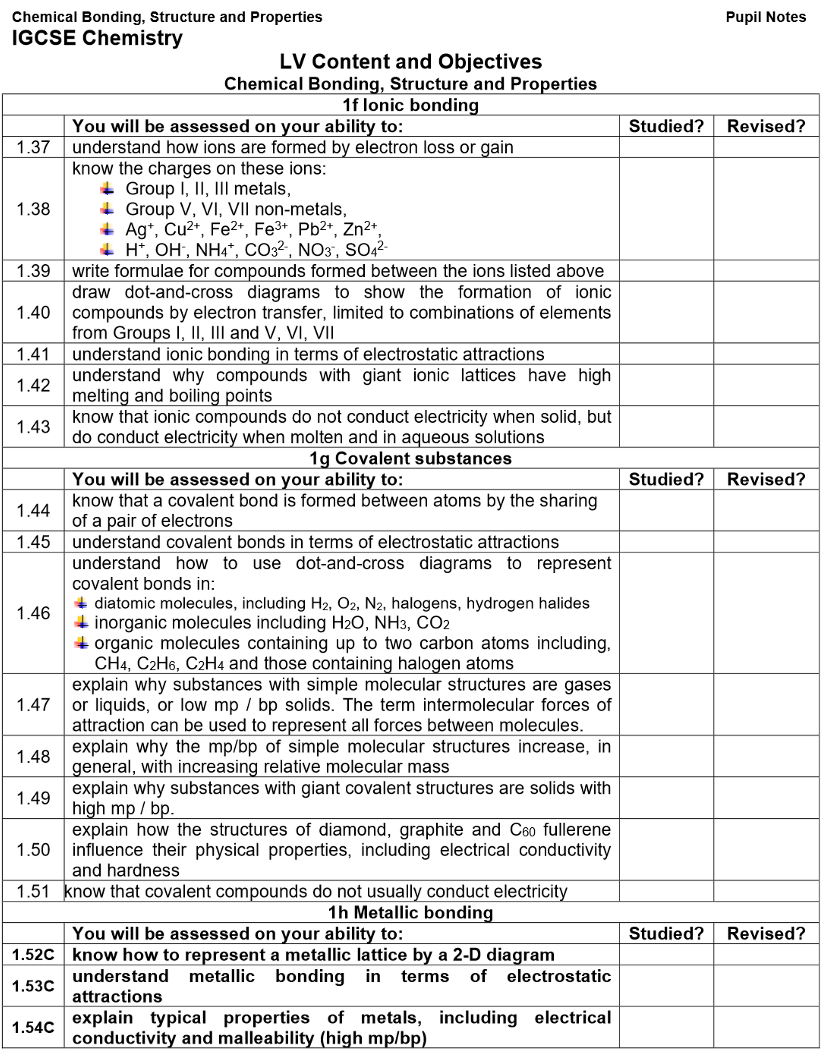
Formulae of Ions:
Atoms lose/gain electrons to get a full outer shell.
The group number shows the number of electrons in the outer shell.
The ion’s charge usually matches the group number:
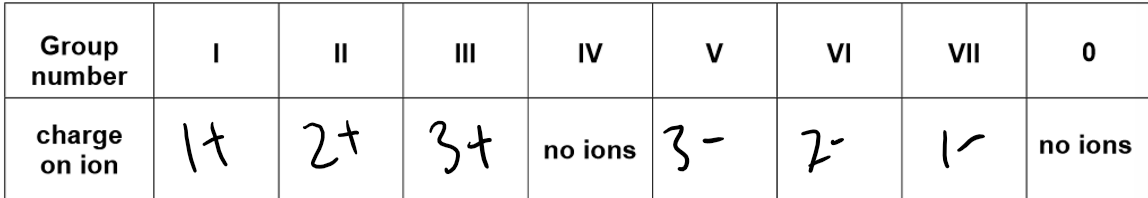
Dot and cross diagrams:
Dot and cross diagrams are used to show the transfer of electrons between metal and non-metal atoms to form positive metal and negative non-metal ions.
Formation of sodium chloride NaCl:
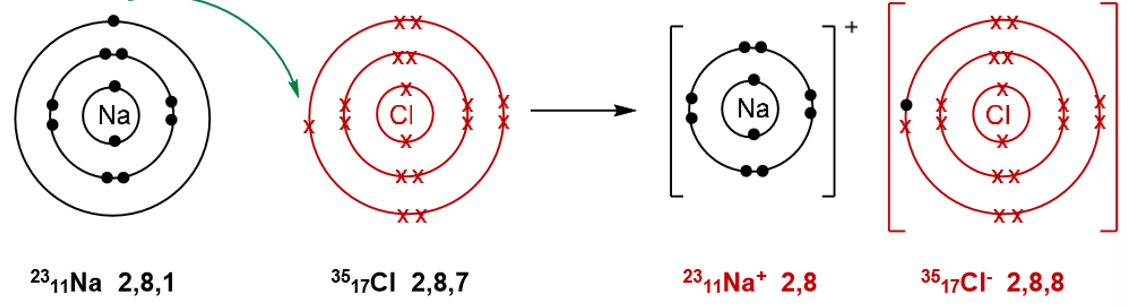
Sodium loses one electron, from the Na+ ion. This electron is transferred to a chlorine atom forming a chloride ion.
Formation of potassium oxide K2O:
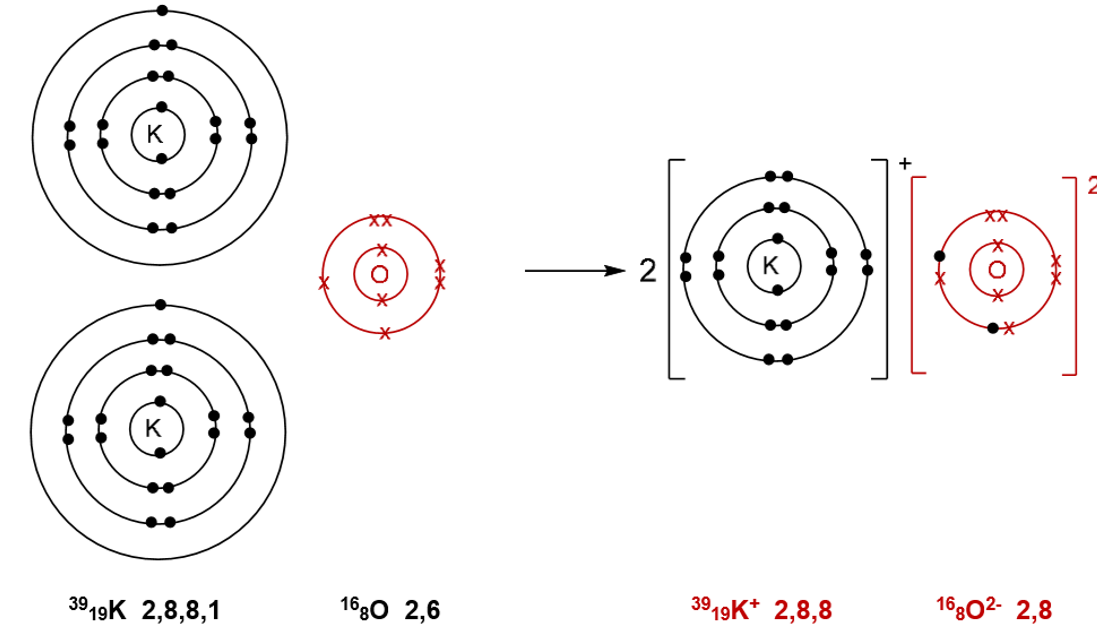
Each potassium atom loses an electron, forming 2 K+ ions. These electrons are transferred to one oxygen atom forming an oxide ion.
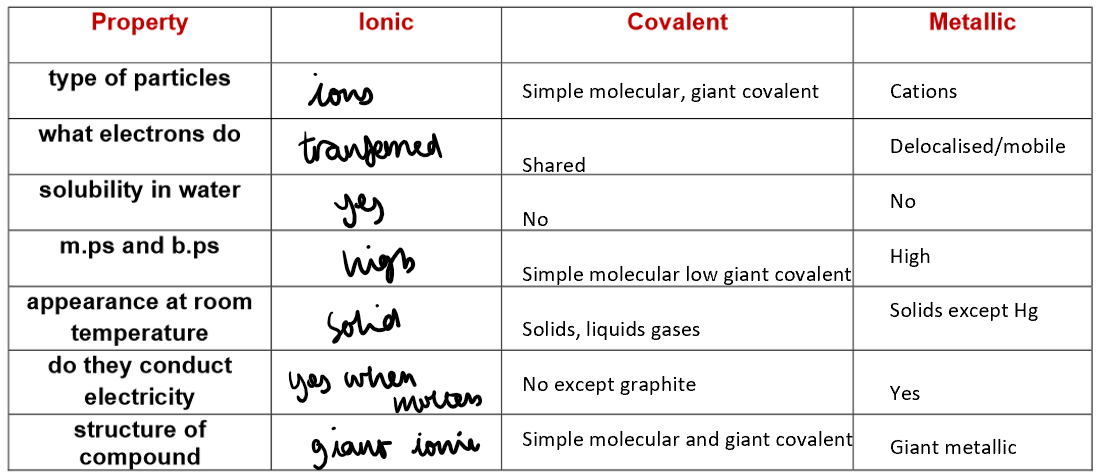
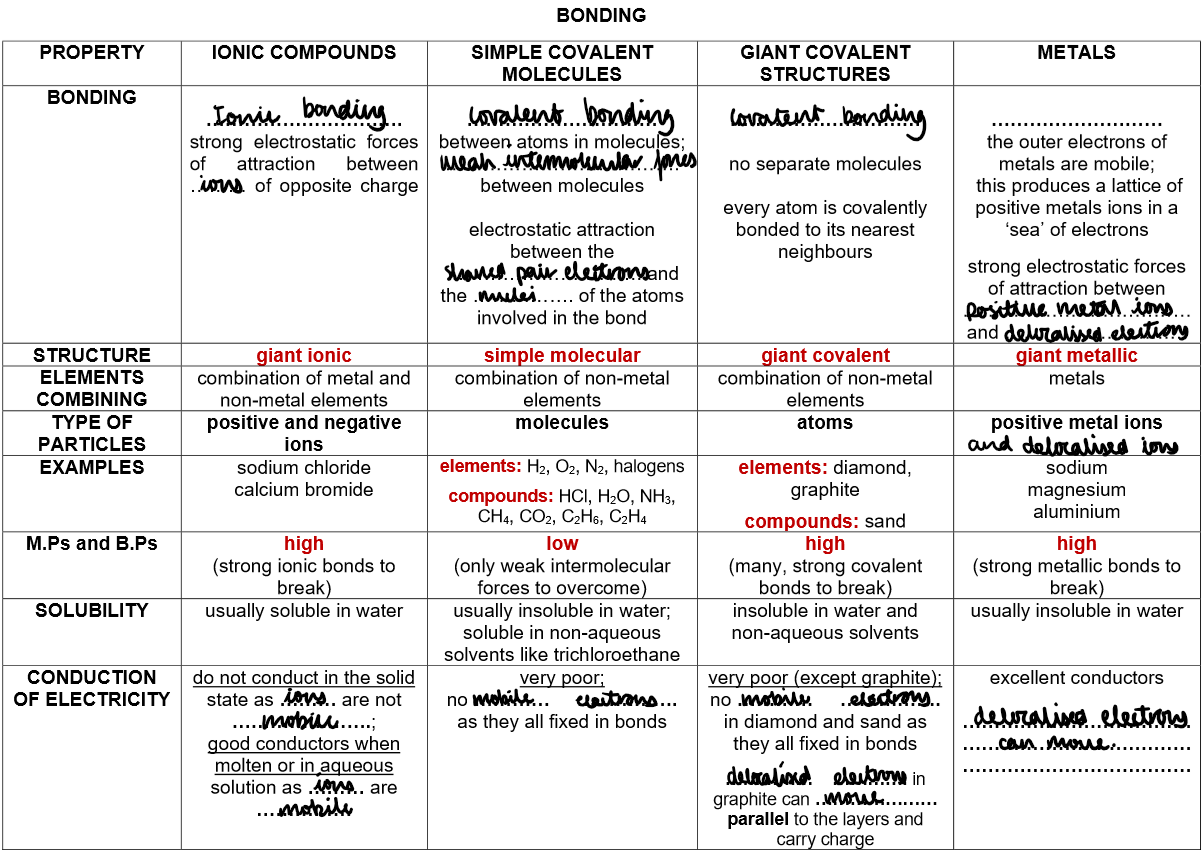
Structure and Properties of Ionic Compounds:
Ionic compounds form a giant ionic lattice of alternating positive and negative ions.
Held together by strong electrostatic forces between oppositely charged ions.
Ionic formulae show the simplest whole-number ratio of ions (empirical formula).
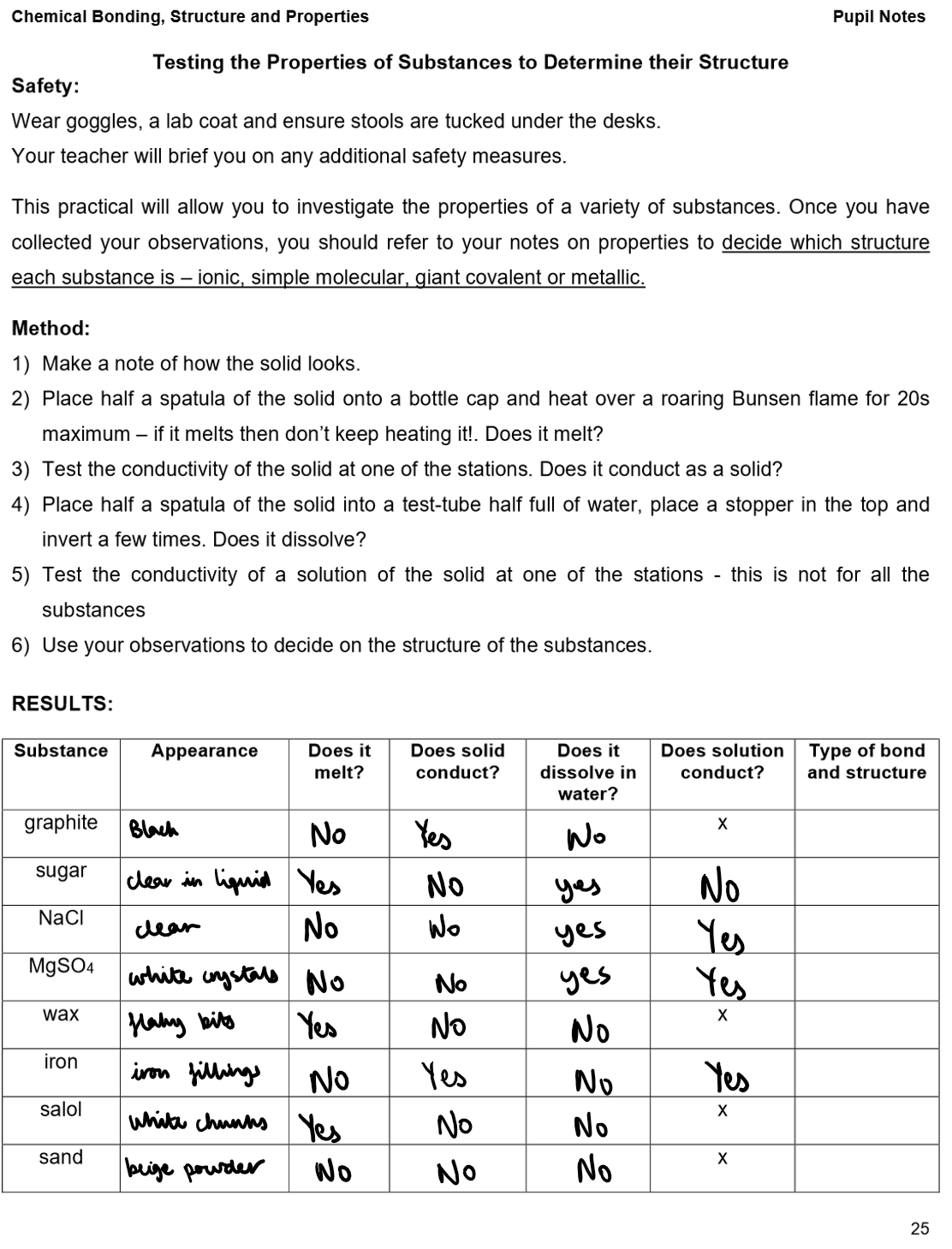
Properties:
High Melting and Boiling Points:
Strong ionic bonds in the lattice need a lot of energy to break, so melting/boiling points are high.
Electrical Conductivity:
Solid: Do not conduct – ions are fixed in place.
Molten or in solution: Do conduct – lattice breaks down, ions are free to move and carry charge (electrolytes).
Magnesium Oxide vs. Sodium Chloride:
MgO has higher charged ions (Mg²⁺, O²⁻) than NaCl (Na⁺, Cl⁻), so stronger bonds.
More energy needed to break these bonds → higher melting point for MgO.
Brittleness:
When the lattice is disturbed, like-charged ions can align.
This causes repulsion, making the crystal split easily.
Electrolysis of lead (II) Bromide:
Safety: This demonstration will be done in a fume cupboard as poisonous vapours are produced.
Method:
The circuit was set up as shown in the diagram below. Notice whether the bulb is lit.
The lead (II) Bromide was heated until it was molten. Notice whether the bold is lit
The power was turned off, the burner removed and the electrodes lifted out of the molten PbBr2.
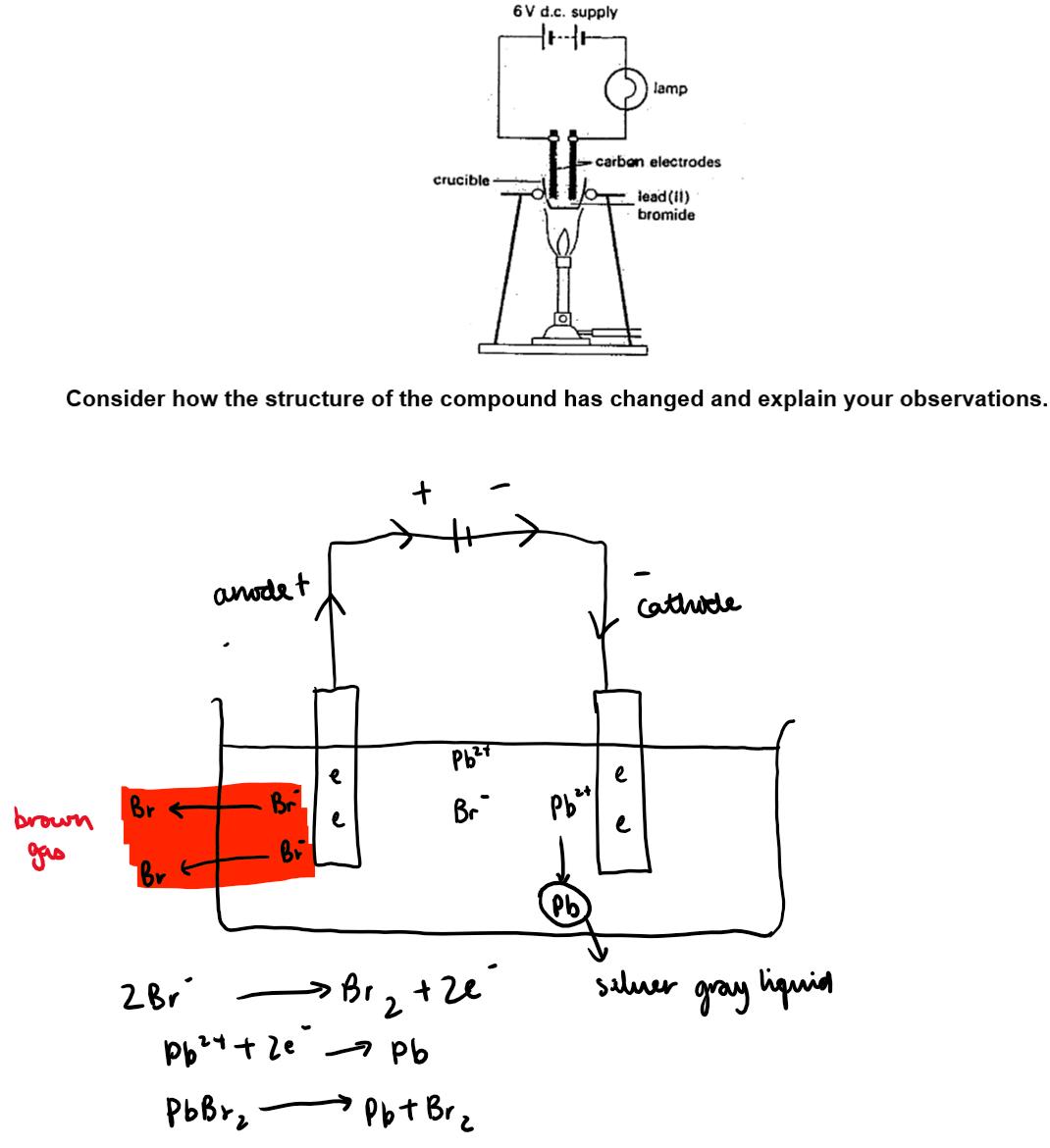
Conclusion:
In solid PbBr₂, Pb²⁺ and Br⁻ ions are fixed in a lattice and cannot move, so no conduction.
When molten, ions become mobile and can carry charge.
Passing an electric current through molten PbBr₂ completes the circuit – bulb lights up
Pb2+ ions move to the cathode, pick up 2 electrons and get deposited as neutral lead atoms (a silvery grey liquid)
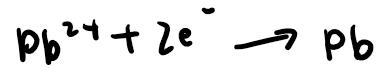
Br ions move to the anode, lose an electron and get deposited as neutral bromine atoms. 2 bromine atoms pair up, to form bromine molecules (brown gas)

Molten PbBr2 undergoes decomposition by an electric current into its elements, Pb and Br 2

Covalent Bonding:
Involves sharing a pair of electrons between two non-metal atoms.
Only outer shell electrons are involved.
A covalent bond is the electrostatic attraction between the shared electrons and both positive nuclei.
One shared pair = single bond.
Two pairs = double bond (e.g. O₂), three pairs = triple bond (e.g. N₂).
Covalent Bonding in Non-Metallic Elements:
This type of bonding leads to the formation of simple molecules.
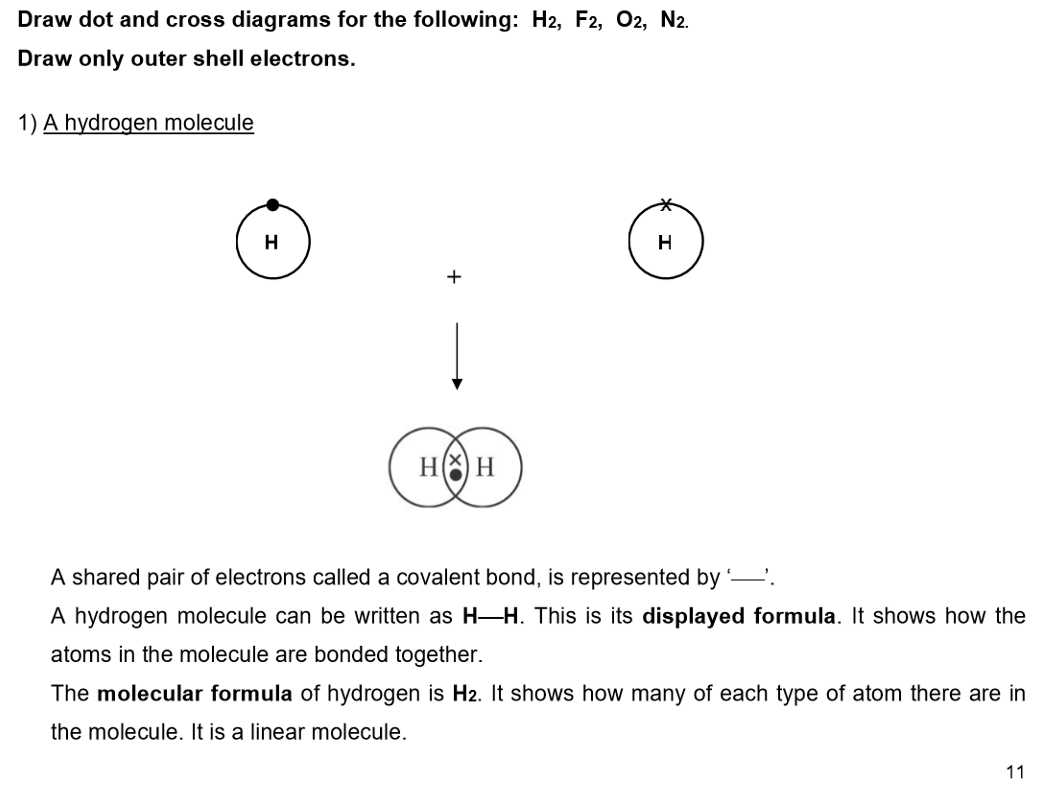
Examples:
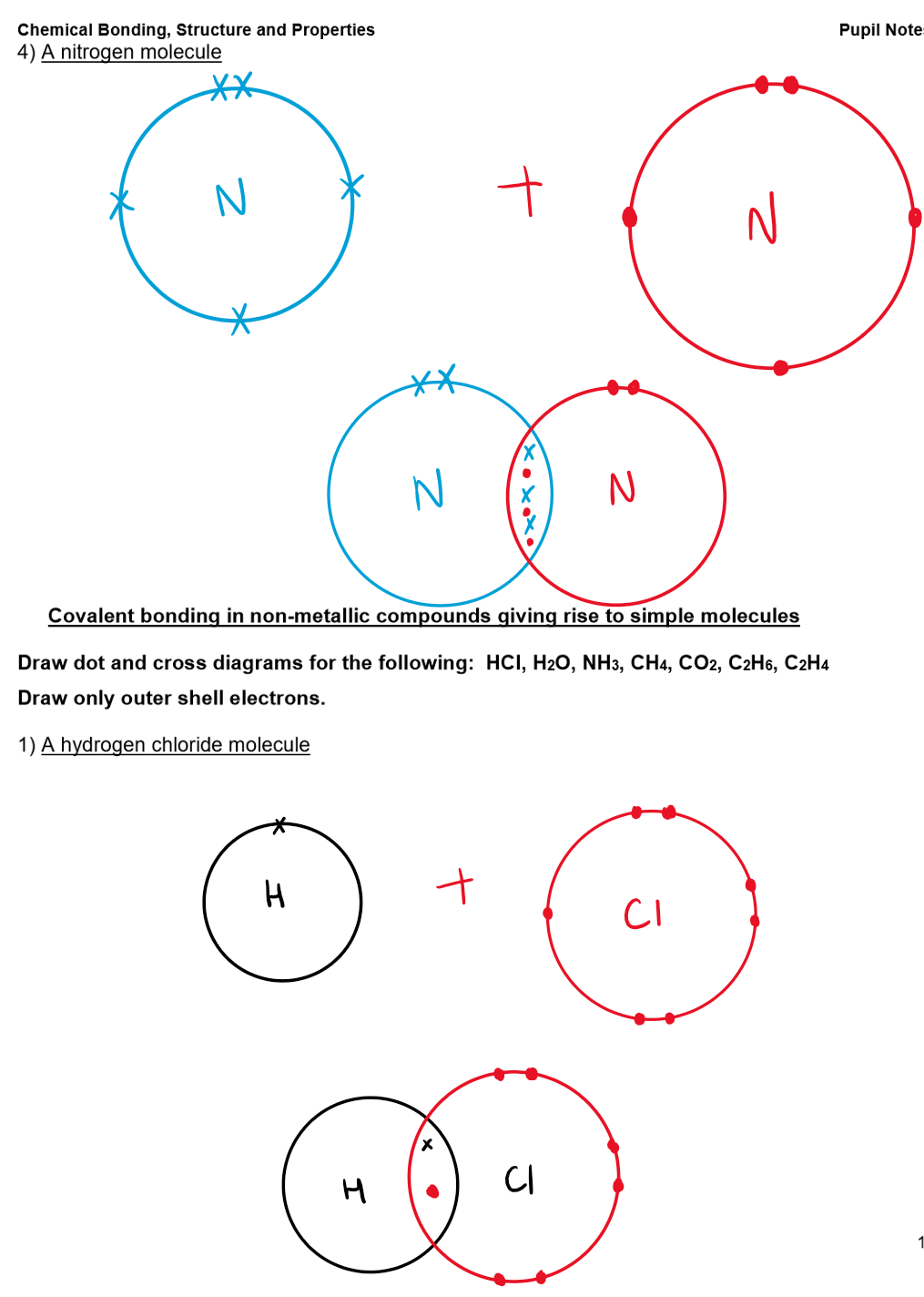
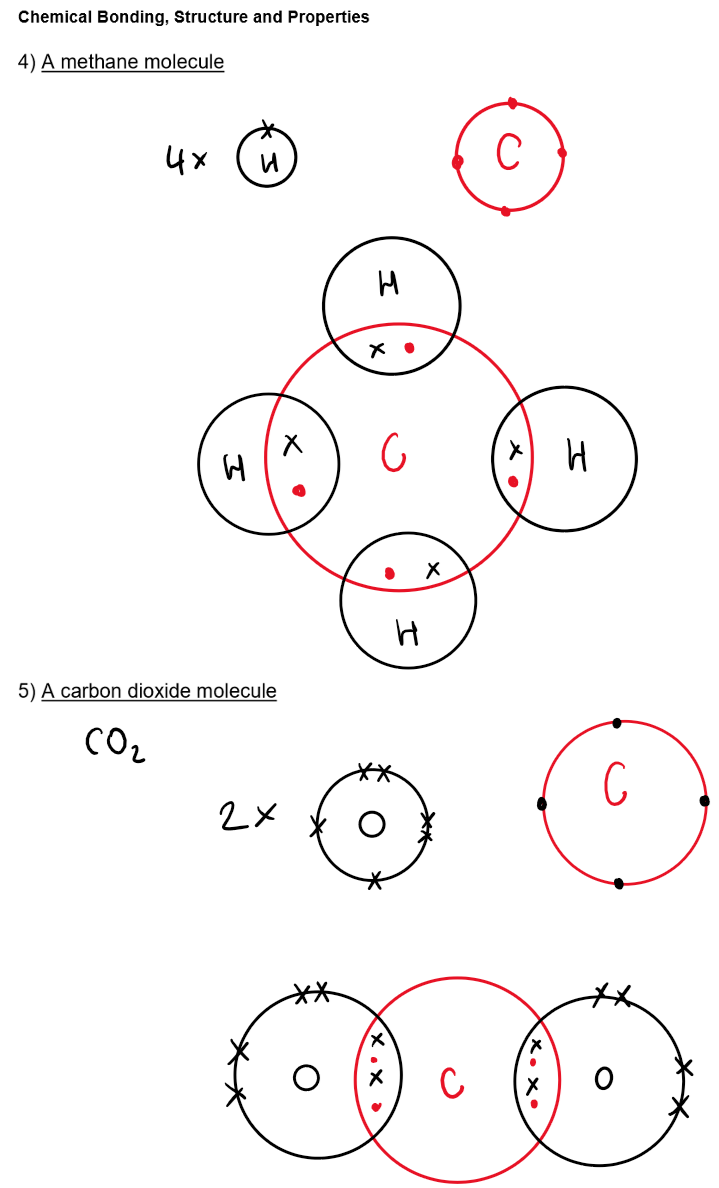
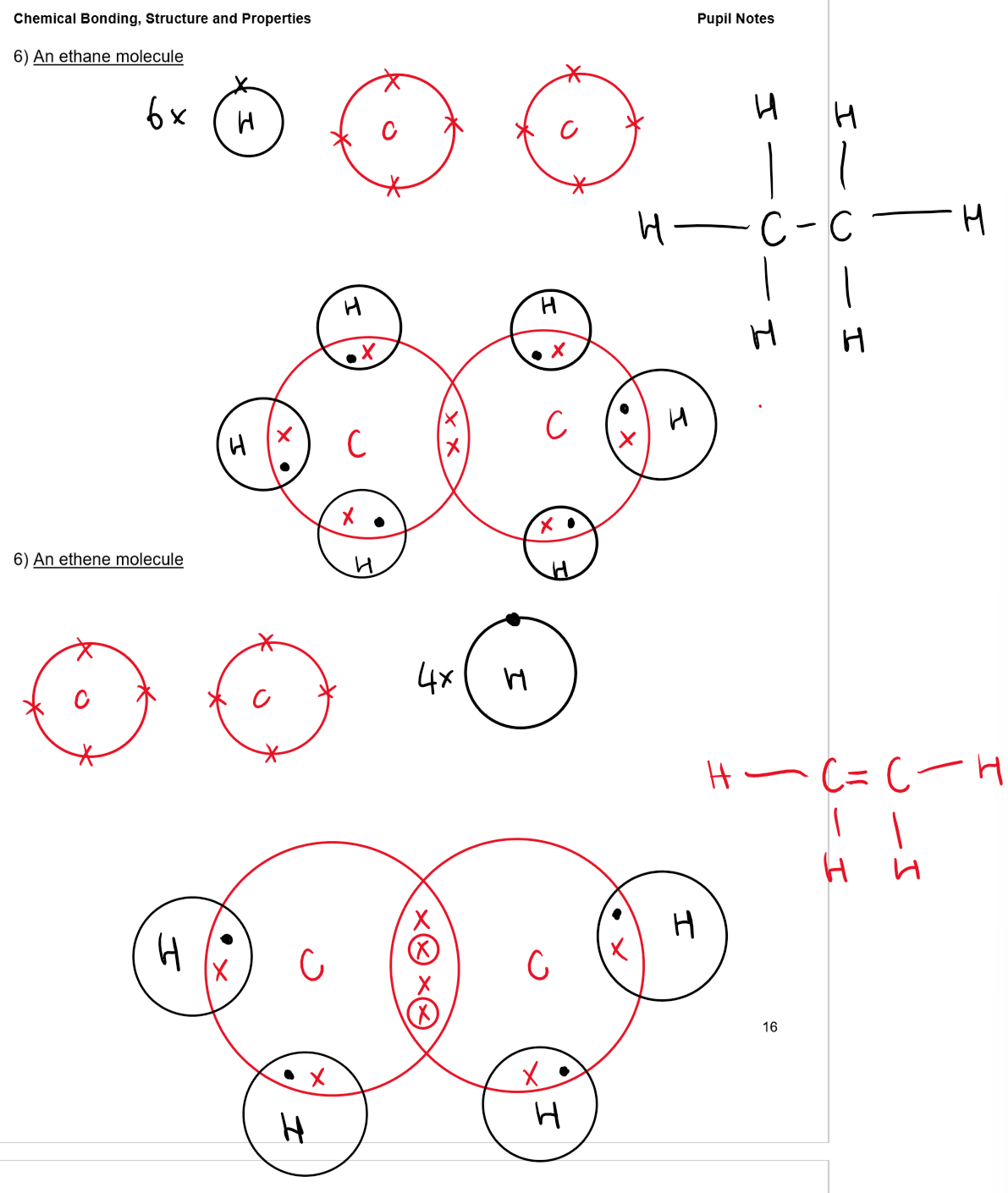
Structure and Properties of Covalent Bonds:
There are two types of covalently bonded structures:
1) Simple Molecular Structures
Examples: H₂, O₂, N₂, C₆₀ fullerene, HCl, H₂O, NH₃, CH₄
Bonding: Strong covalent bonds within molecules
Structure: Simple molecules with weak intermolecular forces between them
Properties:
Low melting/boiling points – little energy needed to overcome weak forces
Do not conduct electricity – no free electrons or ions
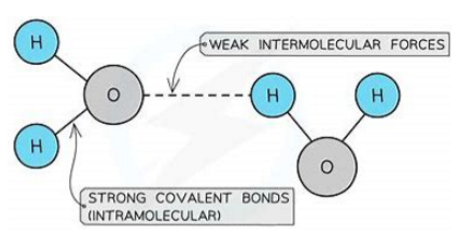
Properties of Simple Molecular Substances:
Low melting and boiling points:
Caused by very weak intermolecular forces between molecules.
These forces require little energy to overcome.
In chemistry, you overcome forces, not break covalent bonds.
Solubility:
Usually insoluble in water, but soluble in non-polar solvents like petrol or trichloroethane.
Exceptions (e.g. HCl, CO₂, SO₂, sugar) may dissolve in water due to interactions with water molecules.
Electrical conductivity:
Do not conduct electricity in any state.
No charged particles, and electrons are fixed in bonds – they cannot move to carry current.
Example questions:
Explain why substances with a simple molecular structure are gases liquids or low m.p/b.p solids:
There are very weak intermolecular forces, between the molecules
Which require little energy to overcome
Explain why the m.p/b.p of simple molecular structures increase, in general, with increasing relative molecular mass:
The larger the relative molecular mass, the bigger and heavier the molecules
Molecules have a greater area of contact, and stronger intermolecular forces
More energy is needed to overcome these forces
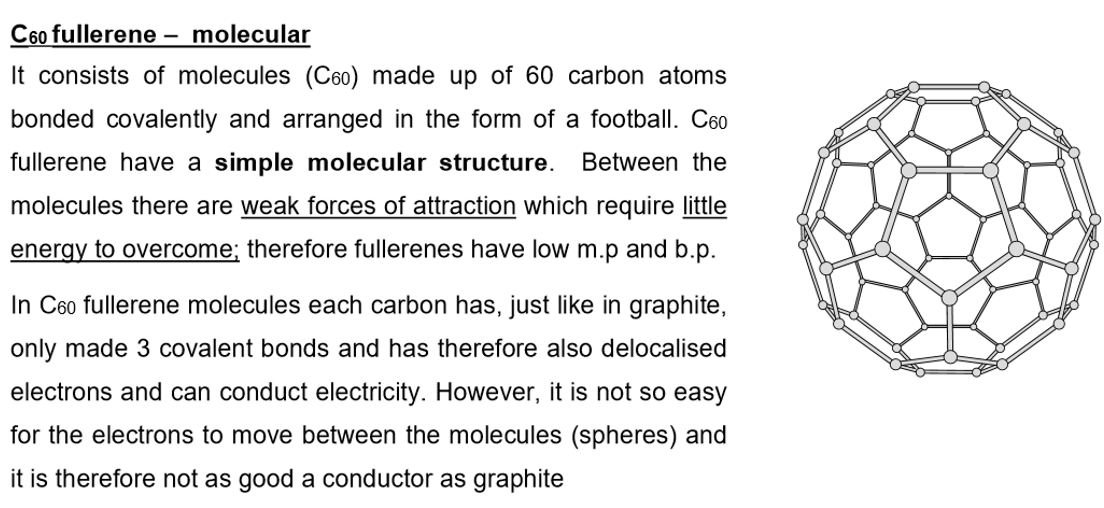
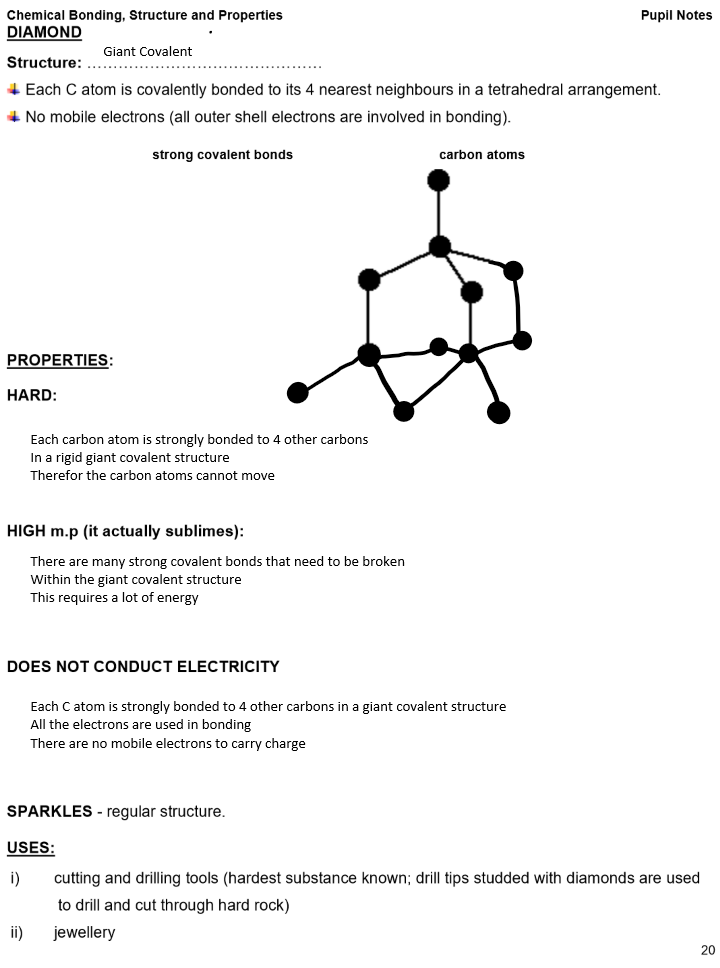
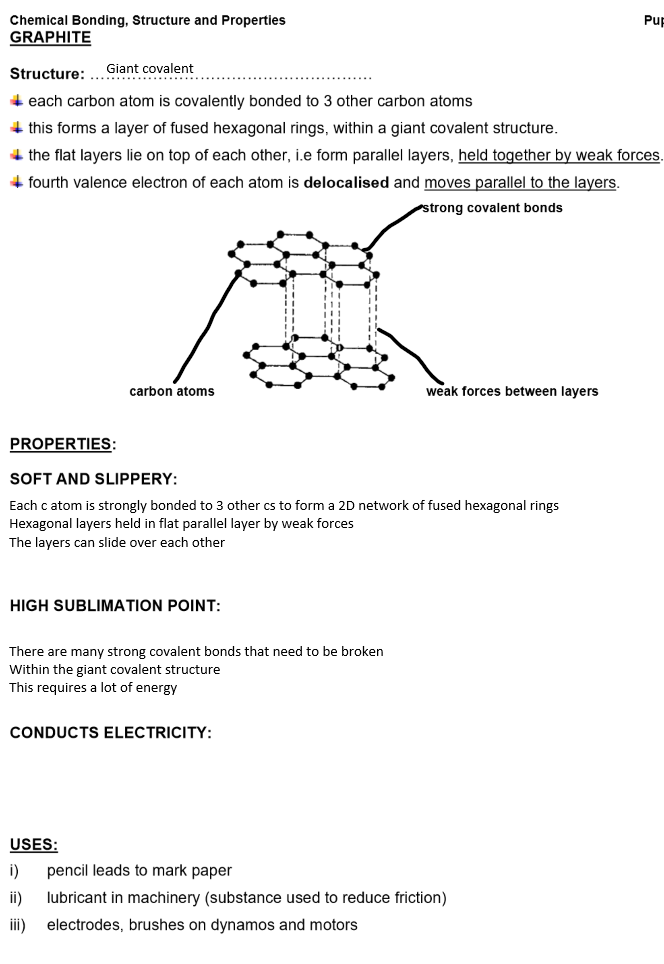
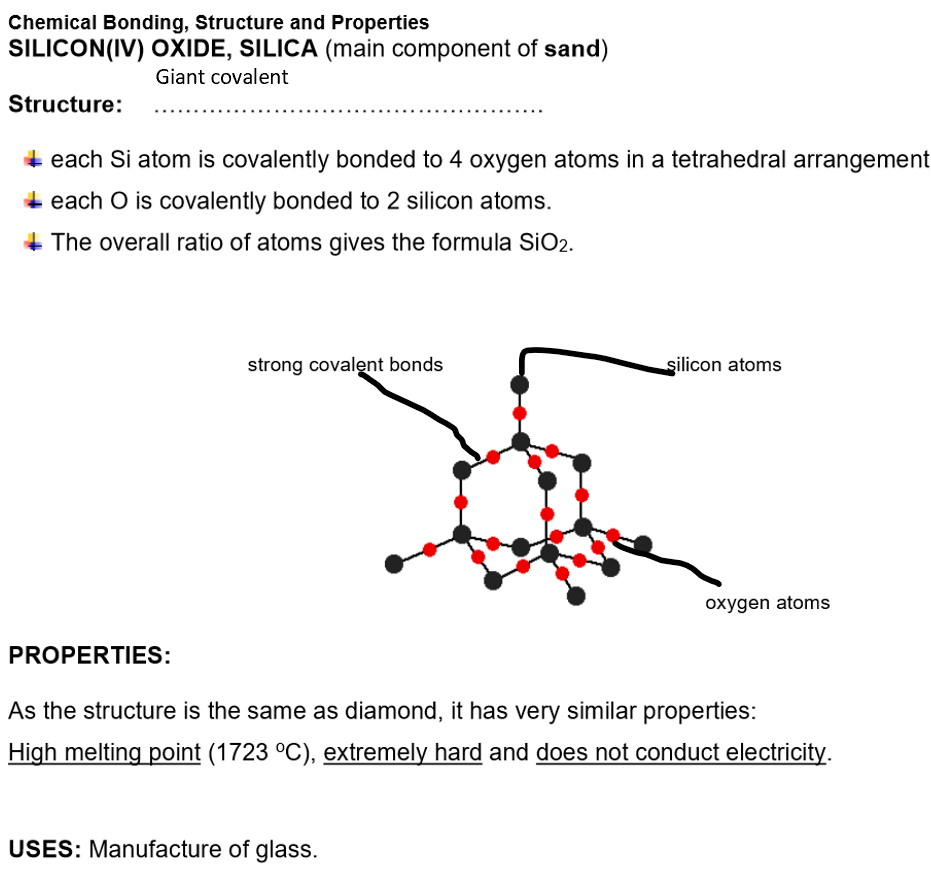
Giant covalent structures e.g. diamond, graphite, sand
Bonding: Strong covalent bonds between all atoms
Structure: Giant covalent with only covalent bond between all atoms in the structure
Properties of giant covalent compounds:
Extremely high melting and boiling points
Many strong covalent bonds need to be broken
Within the giant covalent structure
This requires a lot of energy
Insoluble in all solvents
Examples of giant covalent structures:

Metallic bonds:
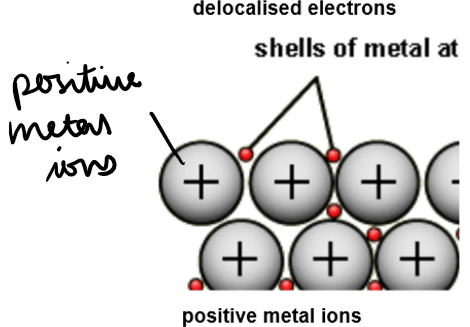
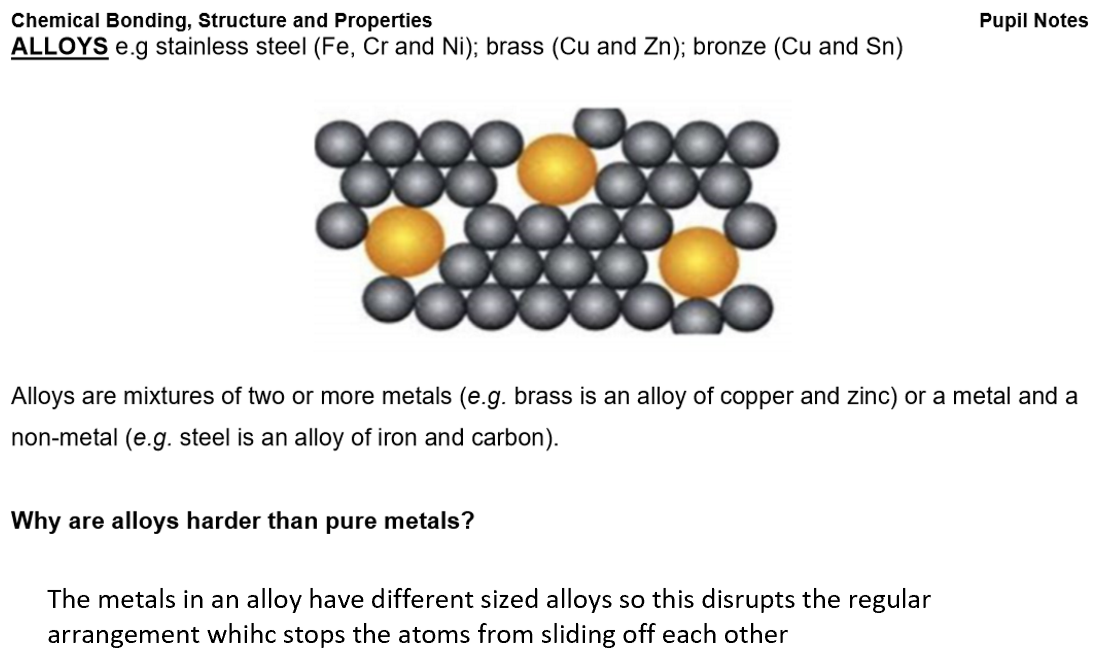
Metallic Bonding:
Structure: Giant metallic lattice – positive metal ions in a regular pattern, surrounded by a sea of delocalised electrons.
Bonding: Strong electrostatic attraction between metal ions and delocalised electrons.
Each metal atom loses outer electrons, forming positive ions, while electrons become free to move.
Properties of Metals:
High melting/boiling points:
Strong metal–electron attraction needs a lot of energy to break.
Good conductors of electricity:
Delocalised electrons move freely and carry charge.
Malleable and ductile:
Layers of ions can slide, so metals can be bent or drawn into wires.
Good conductors of heat:
Mobile electrons transfer thermal energy quickly.
Strong and sonorous:
Can withstand force and produce a ringing sound when struck.

Bonding summary:
Ionic bonding:
The strong electrostatic attractions between the oppositely charged ions e.g. NaCl.
The ionic bond forms due to the transfer of one or more electrons from a metal atom to a non-metal atom which results in oppositely charged ions
Covalent bonding:
The strong electrostatic attraction between the bonding pair of electrons and the nuclei of the atoms involved in the bond
Covalent substances are formed by the sharing pairs of electrons between atoms of the same and/or different non-metal elements
Metallic bonding:
The strong electrostatic attractions between the positively charged metal ions and the delocalised electrons e.g. Cu