History_of_the_atom
Page 1: Solid Sphere Model
John Dalton, early 1800s
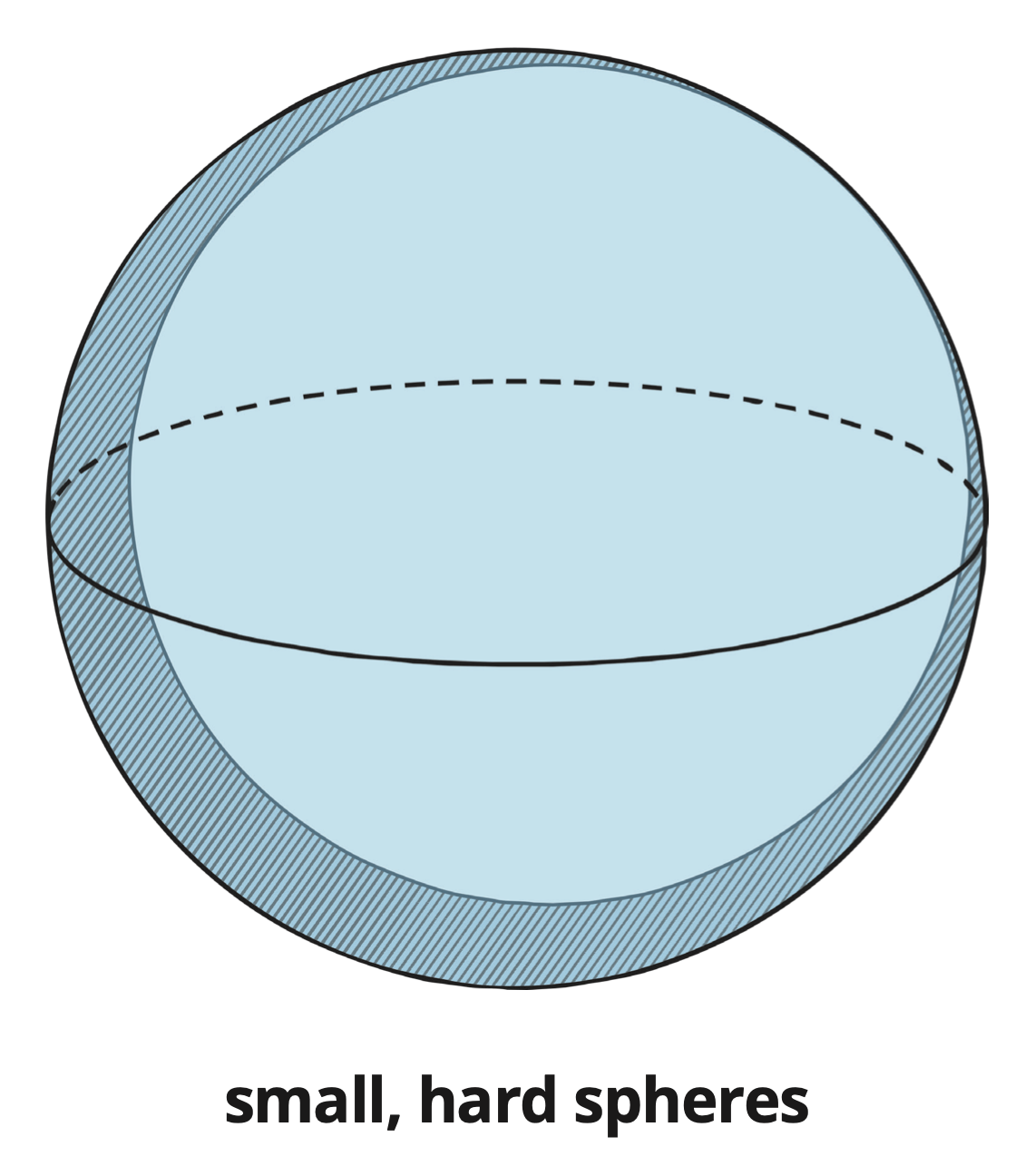
Suggested that substances are made of small hard spheres called atoms.
Proposed that atoms are indivisible (cannot be split into anything smaller).
New substances are formed through the addition and rearrangement of atoms.
Ideas remain useful today, with models being used to describe particle arrangements in solids, liquids, and gases.
Key Concepts:
Atoms: Hard spheres.
States of Matter: Solid, Liquid, Gas.
Page 2: The Plum Pudding Model
J J Thomson, early 1900s
Discovered the electron through extensive experiments.
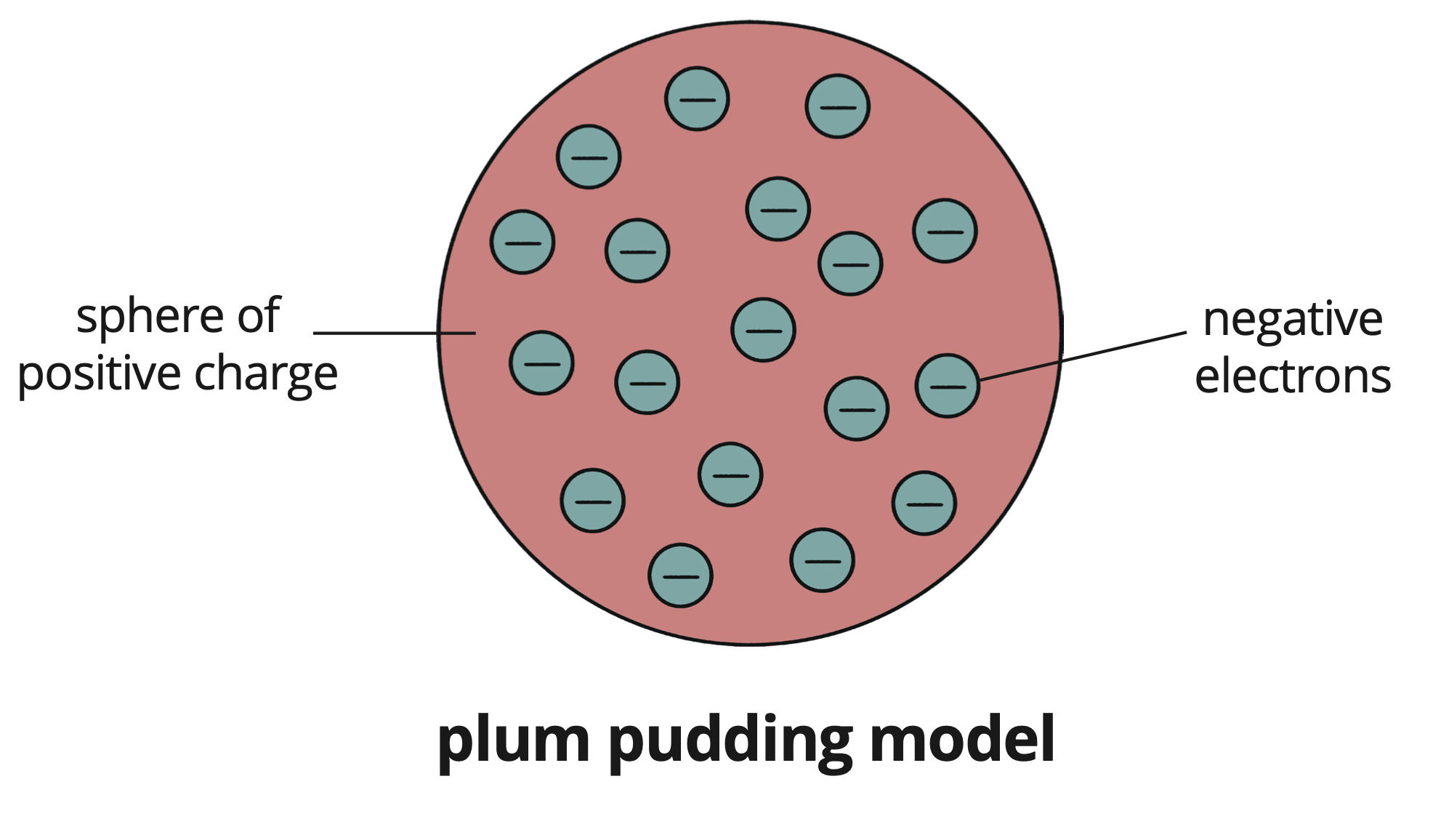
Modified the solid sphere model to propose that atoms consist of a sphere of positive charge with negatively charged electrons embedded within it.
Named this the plum pudding model, likening it to currants in a Christmas pudding.
Key Concepts:
Structure: Sphere of positive charge with embedded negatives (electrons).
Analogy: Christmas pudding.
Page 3: Nuclear Model
Ernest Rutherford, 1909 - 1911
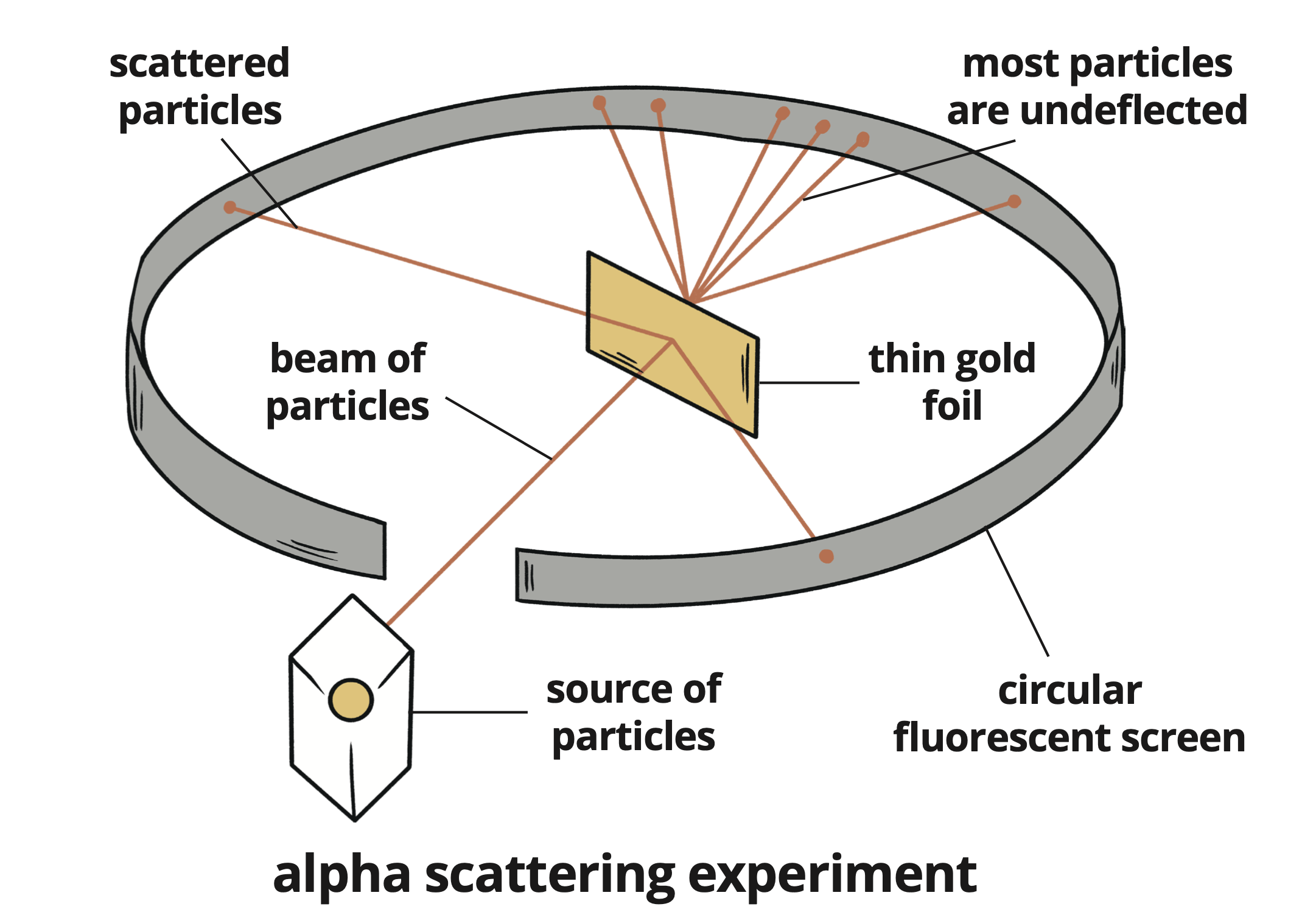
Tested the plum pudding model using an experiment where alpha particles were fired at thin gold foil.
Most particles passed through without deflection, indicating a lot of empty space within the atom.
A small number were scattered in different directions, leading to significant conclusions:
The mass of the atom is concentrated in a central region called the nucleus.
The nucleus carries a positive charge due to the existence of smaller particles named protons.
This nuclear model replaced the plum pudding model.
Key Concepts:
Experiment: Alpha scattering experiment.
Observations: Most particles undeflected, some scattered.
Discovery: Presence of nucleus and protons.
Page 4: Planetary Model
Niels Bohr, 1913
Contributions from theoretical calculations led to insights about electron arrangements.
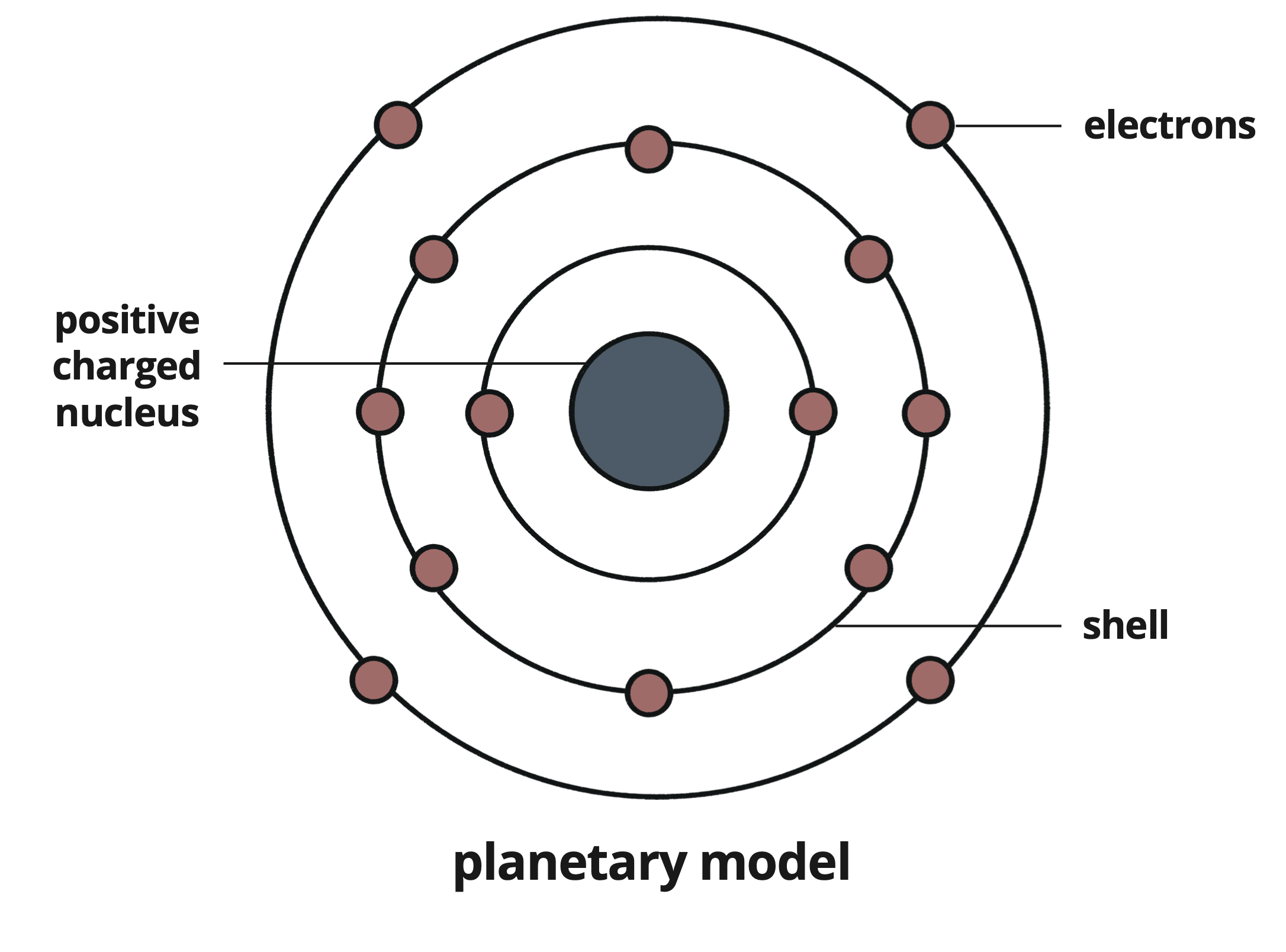
Proposed that electrons orbit the nucleus within defined shells at fixed distances.
Known as the planetary model, this understanding arose from experiments observing light emitted from heated atoms.
Key Concepts:
Structure: Electrons orbiting in shells around a positive nucleus.
Inspiration: Light emissions from heated atoms.
Page 5: Atomic Model
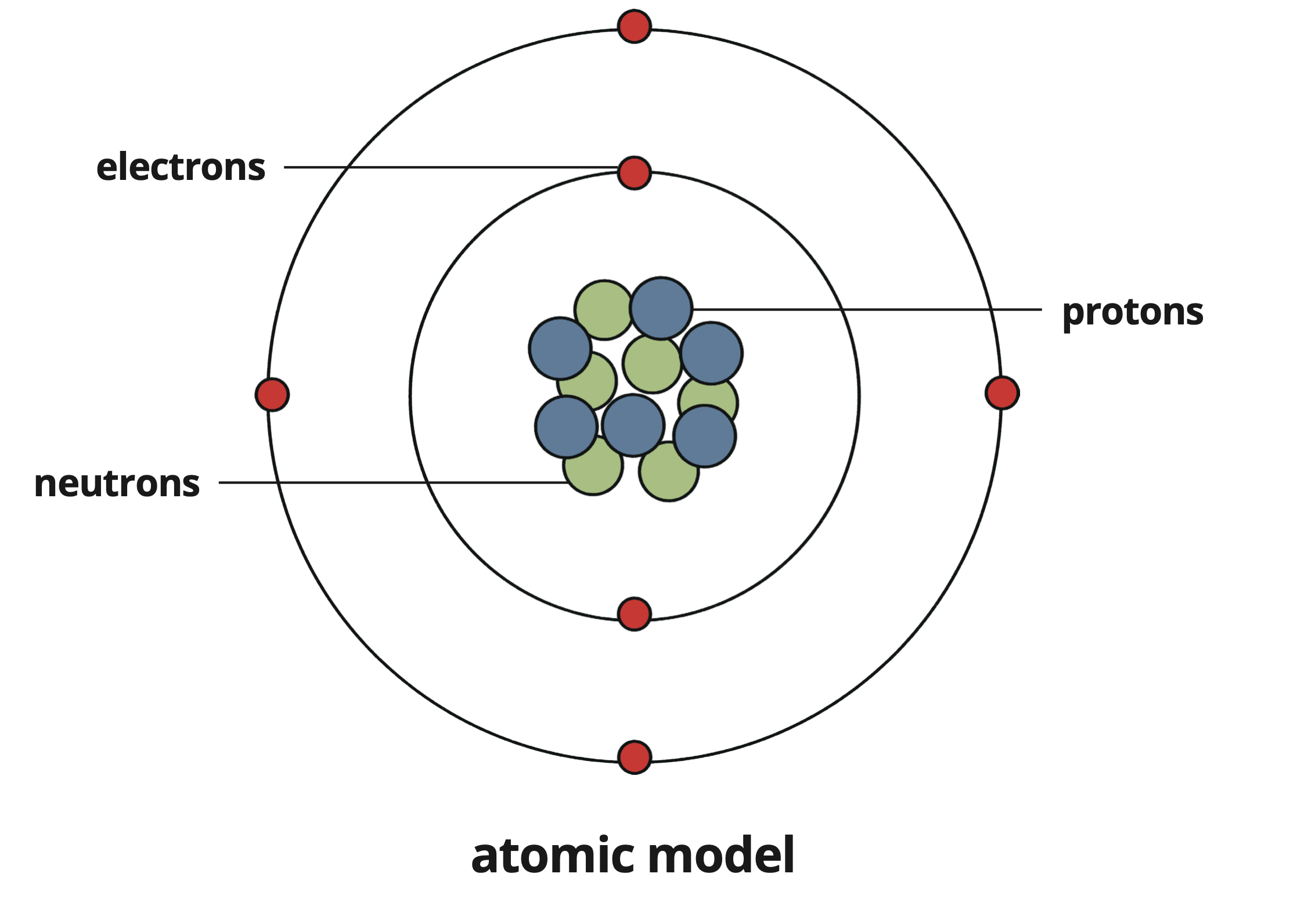
James Chadwick, 1932
Conducted experiments providing evidence for the existence of neutrons, which occupy the nucleus alongside protons.
This atomic model (neutrons + protons in the nucleus with electrons in orbit) is the current accepted model of atomic structure.
Key Concepts:
Structure: Neutrons and protons in the nucleus; electrons in orbit.
Confirmation: Neutron's existence solidified the atomic model.