Mineralogy Notes
2.1 Minerals: Building Blocks of Rocks
Mineralogy: The study of minerals, which are the building blocks of rocks.
Humans have used minerals for practical and decorative purposes for thousands of years.
Quartz: A common mineral, is the source of silicon for computer chips.
Flint and Chert: The first Earth materials mined, fashioned into weapons and cutting tools.
Hematite: A mineral from which iron was extracted, marking the decline of the Bronze Age.
Defining a Mineral
Geologists' Definition: naturally occurring inorganic solid with an orderly crystalline structure + definite chemical composition that allows for some variation.
Characteristics of Minerals:
Naturally Occurring: Formed by natural geologic processes, not synthetic materials.
Generally Inorganic: Inorganic crystalline solids are minerals(halite), but organic (carbon containing) compounds are generally not.
Solid Substance: Only solid crystalline substances are minerals; ice is a mineral, but liquid water and water vapor are not.
Orderly Crystalline Structure: Atoms (or ions) are arranged in an orderly, repetitive manner, forming crystals.
Definite Chemical Composition: Most minerals have a chemical formula (e.g., quartz is SiO_2), but some allow for compositional variation within defined limits.
variation can occur since certain elements can substitute for others of similar size without changing mineral internal structure
Rocks vs. Minerals: Rocks are solid masses of mineral or mineral-like matter that occur naturally.
Most rocks are aggregates of several different minerals joined in a way that retains their individual properties.
Limestone: Composed almost entirely of the mineral calcite.
Nonmineral Matter: Some rocks, like obsidian, pumice, and coal, are composed of nonmineral matter.
2.2 Atoms: Building Blocks of Minerals
Atoms: The smallest particles that constitute specific elements and cannot be split by chemical means.
Composed of protons, neutrons (in the nucleus), and electrons.
Protons and Neutrons: Dense particles with almost identical masses.
Electrons: Negligible mass (about 1/2000 that of a proton).
Electrical Charge:
Protons: +1 charge.
Electrons: -1 charge.
Neutrons: No charge.
Electrically Neutral Substances: Typically contain equal numbers of protons and electrons.
Electron Arrangement: Move about the nucleus in regions called principal shells, each with an associated energy level.
Valence Electrons: Located in the outermost shell and can be transferred or shared with other atoms to form chemical bonds.
Element Definition: Atoms with the same number of protons have the same chemical and physical properties.
Atomic Number: The number of protons in the nucleus of an atom, determining the atom’s chemical nature.
Periodic Table: Organizes elements with similar properties in columns (groups), including atomic number and atomic mass.
Elements and Compounds
Most atoms in the universe (except hydrogen and helium) were created inside massive stars by nuclear fusion and then released into interstellar space during hot, fiery supernova explosions.
As this ejected material cooled, the newly formed nuclei attracted electrons to complete their atomic structure.
At the temperatures found at Earth’s surface, free atoms (those not bonded to other atoms) generally have a full complement of electrons—one for each proton in the nucleus.
Elements: Defined by the number of protons.
Chemical Compounds: Most minerals are chemical compounds composed of atoms of two or more elements (e.g., quartz, SiO_2; halite, NaCl).
Single-Element Minerals: A few minerals, like diamonds, sulfur, and native gold and copper, are made entirely of atoms of only one element.
2.3 Why Atoms Bond
Chemical Bonding: Atoms bond to achieve stability, typically by attaining a full valence shell of electrons like noble gases.
Octet Rule: Atoms tend to gain, lose, or share electrons until surrounded by eight valence electrons (useful guideline but has exceptions).
Chemical Bond Definition: The transfer or sharing of electrons to attain a full valence shell.
Ionic Bonds: Electrons are transferred between elements to form ions (positively and negatively charged atoms).
Covalent Bonds: Electrons are shared between atoms.
Metallic Bonds: Valence electrons are shared among all atoms in a substance.
Types of Chemical Bonds
Ionic Bonds:
One atom gives up one or more valence electrons to another, forming ions.
The atom that loses electrons becomes a positive ion (cation), and the atom that gains electrons becomes a negative ion (anion).
Oppositely charged ions attract and form ionic compounds.
Example: Sodium chloride (NaCl), where sodium gives up an electron to chlorine.
Covalent Bonds:
Chemical bond formed by the sharing of one or more valence electrons between a pair of atoms.
No ions are present.
Example: Hydrogen molecule (H_2), where electrons are shared between two hydrogen atoms.
Metallic Bonds:
Atoms contribute valence electrons to a common pool of electrons that freely move throughout the metallic structure.
Creates an array of positive ions immersed in a “sea” of valence electrons.
Responsible for metals' unique properties: good conductors of electricity, malleable (hammered into sheets), and ductile (drawn into wires).
2.4 Properties of Minerals
Luster: Appearance or quality of light reflected from the surface of a mineral.
Metallic: Shiny like a metal (e.g., galena).
Submetallic: Dull coating or tarnish (e.g., tarnished copper).
Nonmetallic: Described as vitreous (glassy), dull or earthy, pearly, silky, or greasy.

Color: Considered a diagnostic property for only a few minerals due to variations caused by slight impurities.

Streak: Color of a mineral in powdered form, obtained by rubbing it across a streak plate (unglazed porcelain).
Metallic minerals generally have a dense, dark streak, while nonmetallic minerals have a light-colored streak.

Ability to Transmit Light:
Opaque: No light is transmitted.
Translucent: Light, but not an image, is transmitted.
Transparent: Both light and an image are visible.
Crystal Shape, or Habit:
Characteristic shape of individual crystals or aggregates of crystals.
Examples: Equant (equidimensional), bladed, fibrous, tabular, cubic, prismatic, platy, blocky, and banded.

Mineral Strength
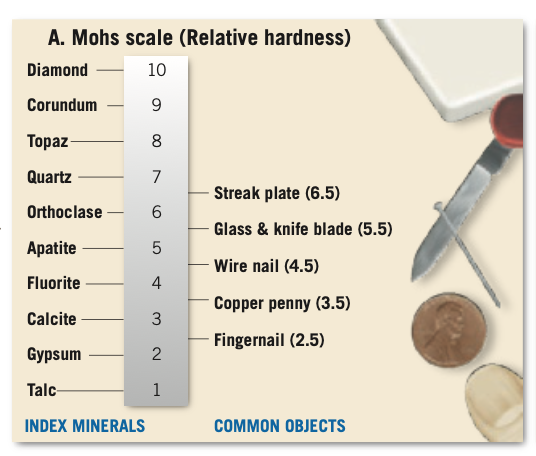
Hardness: Measure of a mineral's resistance to abrasion or scratching.
Mohs Scale of Hardness: Consists of 10 minerals arranged from 1 (softest) to 10 (hardest).
Fingernail: About 2.5.
Copper penny: About 3.5.
Glass: About 5.5.
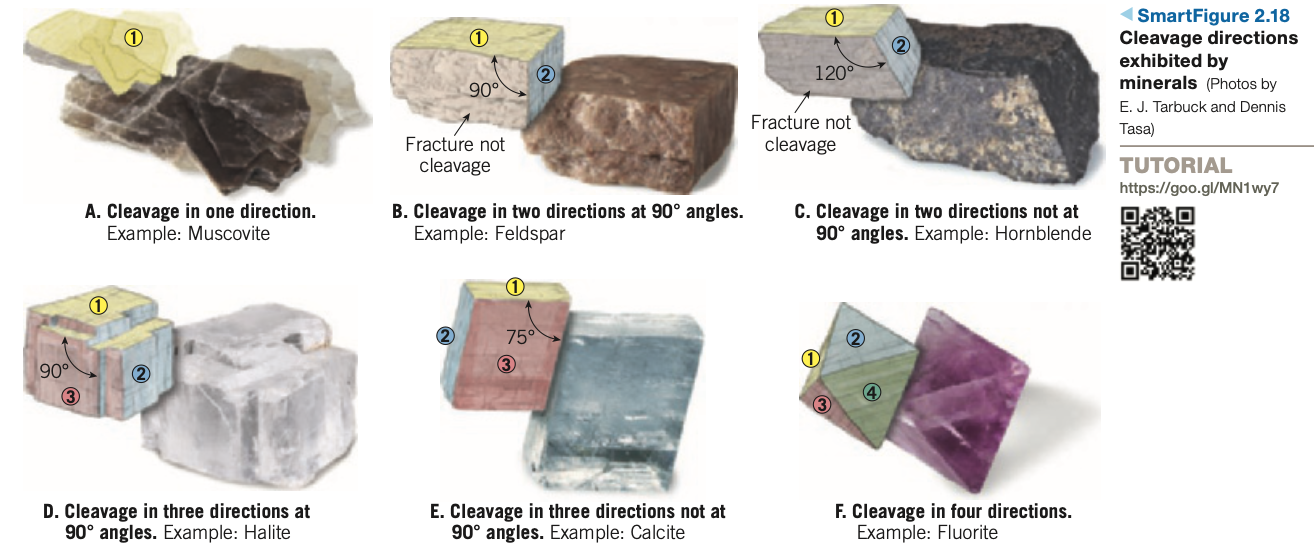
Cleavage: Tendency of a mineral to break along planes of weak bonding, producing smooth, flat surfaces.
Described by the number of cleavage directions and the angles at which they meet.

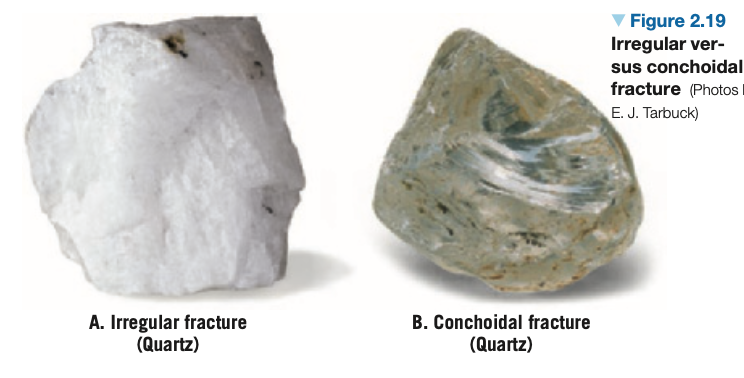
Fracture: Occurs in minerals with chemical bonds that are equally, or nearly equally, strong in all directions.
Irregular fracture: Produces uneven surfaces.
Conchoidal fracture: Produces smooth, curved surfaces resembling broken glass.
Tenacity: Describes how a mineral responds to stress (e.g., brittle, malleable, sectile, elastic).
Density and Specific Gravity
Density: Mass per unit volume.
Specific Gravity: Ratio of a mineral’s weight to the weight of an equal volume of water.
Most common minerals have a specific gravity between 2 and 3.
Metallic minerals (e.g., galena, gold) have higher specific gravities.
Other Properties of Minerals
Taste: E.g., Halite tastes salty.
Feel: Talc feels soapy, graphite feels greasy.
Smell: Streaks of some sulfur-bearing minerals smell like rotten eggs.
Magnetism: Magnetite is magnetic.
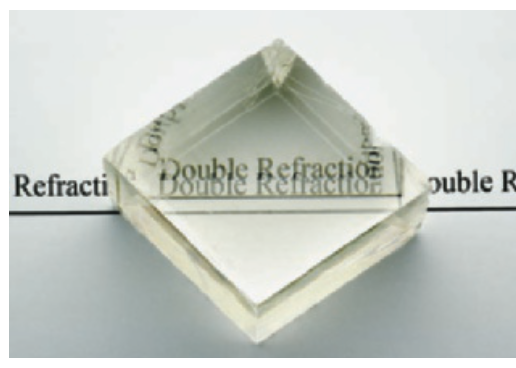
Double Refraction: Transparent calcite placed over text makes the letters appear twice.
Chemical Test: Carbonate minerals effervesce (fizz) when dilute hydrochloric acid is applied.
Mineral Groups
Rock-Forming Minerals: A few dozen minerals that make up most of the rocks of Earth’s crust.
Economic Minerals: Minerals used extensively in manufacturing.
Eight Most Abundant Elements: Make up the vast majority of rock-forming minerals (oxygen, silicon, aluminum, iron, calcium, sodium, potassium, magnesium).
Silicate Minerals: Contain oxygen and silicon atoms, forming the silicon-oxygen tetrahedron (SiO_4^{4-} ).
Account for more than 90 percent of Earth’s crust.
Nonsilicate Minerals: Less abundant but economically important (e.g., iron, aluminum, gypsum, copper).
Most nonsilicate mineral classes contain members that are prized for their economic value. This includes the oxides, whose members hematite and magnetite are important ores of iron
Silicate Structures
Silicon-Oxygen Tetrahedron: The fundamental building block of silicate minerals, consisting of four oxygen ions covalently bonded to a silicon ion (SiO_4^{4-}).
Tetrahedra Arrangement: Joined into chains, sheets, or three-dimensional networks by sharing oxygen atoms.
Joining Elements: Iron (Fe), magnesium (Mg), potassium (K), sodium (Na), and calcium (Ca).
Feldspars: The most plentiful group, comprising about 51 percent of Earth’s crust.
Quartz: The second-most-abundant mineral in the continental crust, made completely of silicon and oxygen.
Because the silicon–oxygen bonds are strong, silicate minerals tend to cleave between the silicon–oxygen structures rather than across them
Formation of Silicate Minerals
Crystallization from Molten Rock: Cooling at or near Earth’s surface or at great depths.
Weathering: From the weathered products of other silicate minerals (e.g., clay minerals).
Mountain Building: Under extreme pressures, silicate minerals are formed.
Common Silicate Minerals
Light Silicate Minerals
Generally light in color and less dense.
Contain varying amounts of aluminum, potassium, calcium, and sodium.
Feldspar Group: The most plentiful silicate group (51 percent of Earth’s crust).
Two structures: Potassium feldspar (contains potassium ions) and plagioclase feldspar (contains both sodium and calcium ions).
Rectangular shape and smooth, shiny faces.
Salmon-colored feldspar belongs to the potassium feldspar subgroup.
Quartz: Second-most-abundant mineral in the continental crust, consisting of silicon and oxygen.
Hard, resists weathering, and does not have cleavage.
Muscovite: A common member of the mica family with excellent cleavage in one direction.
Clay Minerals: Sheet structure, originate as products of chemical weathering of other silicate minerals.
Kaolinite: Used in fine china and coating for high-gloss paper.
Dark Silicate Minerals
Contain ions of iron and/or magnesium, dark in color, and have a greater specific gravity.
Olivine Group: High-temperature silicate minerals, black to olive green, glassy luster.
Pyroxene Group: Important components of dark-colored igneous rocks (e.g., augite).
Amphibole Group: Chemically complex, hornblende is the most common member.
Biotite: A dark, iron-rich member of the mica family with excellent cleavage.
Garnet: Individual tetrahedra linked by metallic ions, glassy luster, lacks cleavage, and exhibits conchoidal fracture.
Important Nonsilicate Minerals
Nonsilicate Mineral Groups: Typically divided based on the negatively charged ion or complex ion that the members have in common (e.g., oxides, carbonates, sulfates, halides).
Carbonates: Composed of the carbonate ion (CO_3^{2-}) and one or more positive ions (e.g., calcite, dolomite).
Sedimentary Rocks: Halite and gypsum are frequently found in sedimentary rocks.
Economic Value: Many nonsilicates, such as hematite, magnetite, galena, sphalerite, and chalcopyrite, are prized for their economic value.
Minerals: A Nonrenewable Resource
Natural Resources: Grouped into categories based on their renewability or origin/type.
Renewable Resources: Can be replenished over relatively short time spans (e.g., corn, cotton, energy from flowing water, wind, Sun).
Nonrenewable Resources: Form continuously, but the processes are so slow that significant deposits take millions of years to accumulate (e.g., iron, aluminum, copper, petroleum, natural gas, coal).
Mineral Resources: Occurrences of useful minerals formed in quantities that eventual extraction is reasonably certain.
Ore Deposit: A naturally occurring concentration of one or more metallic minerals that can be extracted economically.