Carbon and Functional Groups
Introduction
Course: Biology 189: Fundamentals for Life Sciences
Instructor: Earl Yoon
Topic: Carbon, Functional Groups, and Chemical Reactions
Learning Objectives
Understand the role of carbon as the backbone of organic compounds.
Describe hydrocarbons and organic compounds.
Define isomers and identify them in different compounds.
Identify the seven functional groups (hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, methyl, phosphate) by name, structure, and properties.
Learn to read shorthand chemical structures, identifying all bonded atoms and their arrangements.
General Properties of Macromolecules
Carbon-based with a carbon backbone.
Presence of functional groups, enhancing diversity and functionality.
Carbon in Organic Molecules
Carbon is the foundational element of organic molecules, which typically consist of carbon and hydrogen.
Carbon is the “backbone” of large biologically-related molecules
Why? Carbon atoms have 4 unpaired valence electrons (can form lots of different bond configurations
Why Carbon Forms the Backbone
Carbon's tetravalence allows it to form four bonds with different atoms.
Capable of creating combinations of single, double, and triple bonds.
Catenation: Bonding of carbon atoms occurs due to high bond strength, small atomic size, and moderate electronegativity. (can catenate form chains and rings with itself)
Diversity in Organic Compounds
Carbons can be arranged in a linear structure or ring structures.
Examples:
C8H18 (Octane)
C2H6O (Ethanol)
C6H12O6 (Glucose)
Shapes of Organic Compounds
Length: Varies in carbon chain length.
Branching: Carbons can branch in various configurations.
Double bond position: Affects structure and properties.
Presence of rings: Can alter molecule stability and functionality.
Simplest Organic Compounds
Hydrocarbons: Organic compounds made solely of carbon and hydrogen.
Examples: Methane, Propane, Benzene, Octane
Characteristics: Hydrophobic and release significant energy when broken down.
Isomers
Isomers: Molecules with the same chemical formula but with different arrangement of atoms
Example: C6H12O6 can exist in various forms (e.g., glucose).
Functional Groups of Macromolecules
Functional Group: Chemical group that has specific properties (attached to carbon skeleton of compound)
Functional groups impart unique properties:
Ethane: Nonpolar
Ethanol: Polar, dissolves in water due to -OH group
Propanoic acid: Acidic, dissolves in water
Hydroxyl Group
Hydro- Hydrogen Oxyl-Oxygen
Properties:
Polar
Hydrophilic (forms w water)
Found in:
Alcohols
Monosaccharide sugars
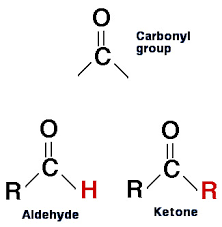
Carbonyl Group
Carbo- Carbon Nyl- Oxygen
Properties:
Polar
Hydrophilic
Found in:
Sugars and other molecules
Types:
Ketone: Carbonyl group located between two carbons
Aldehyde: Carbonyl group at the end of a carbon chain
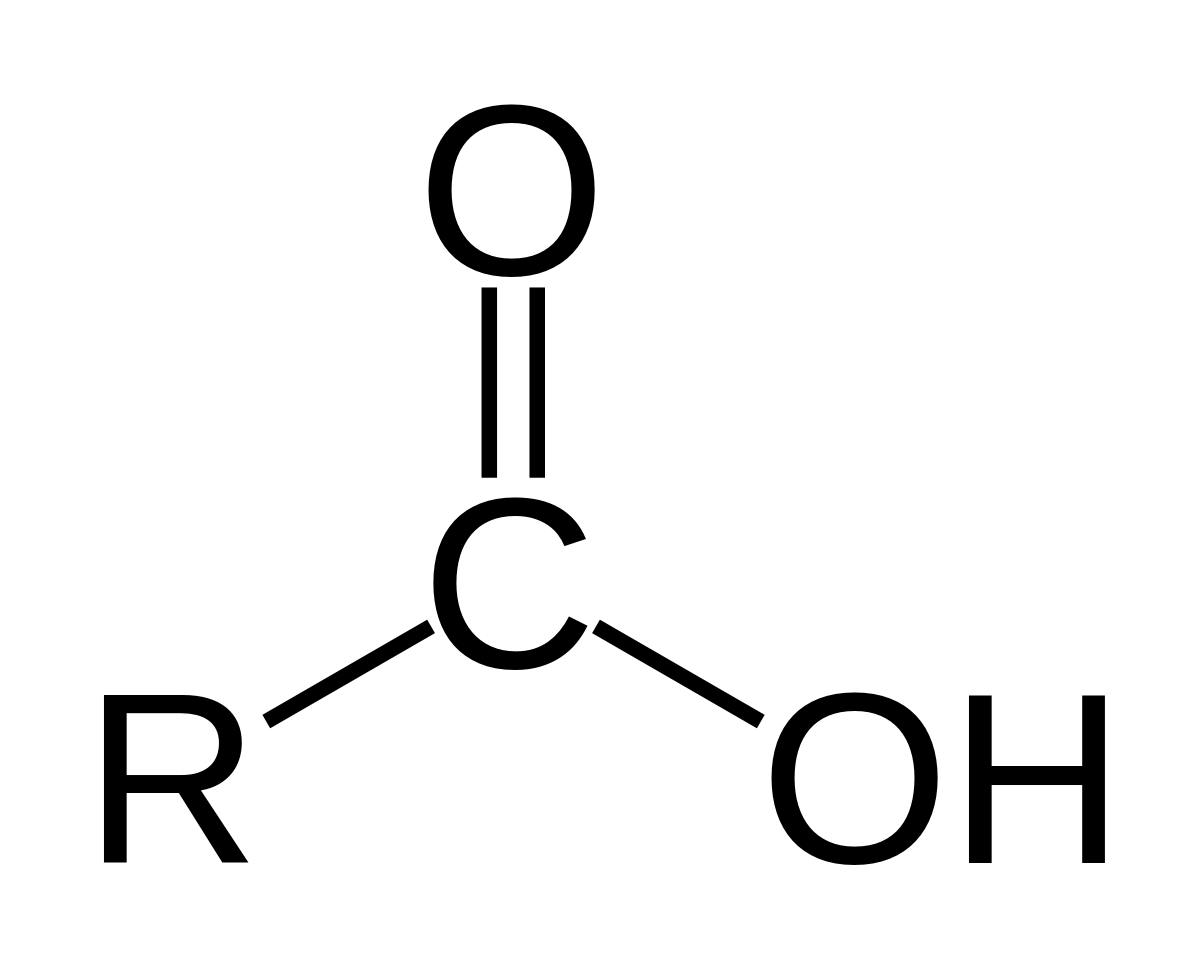
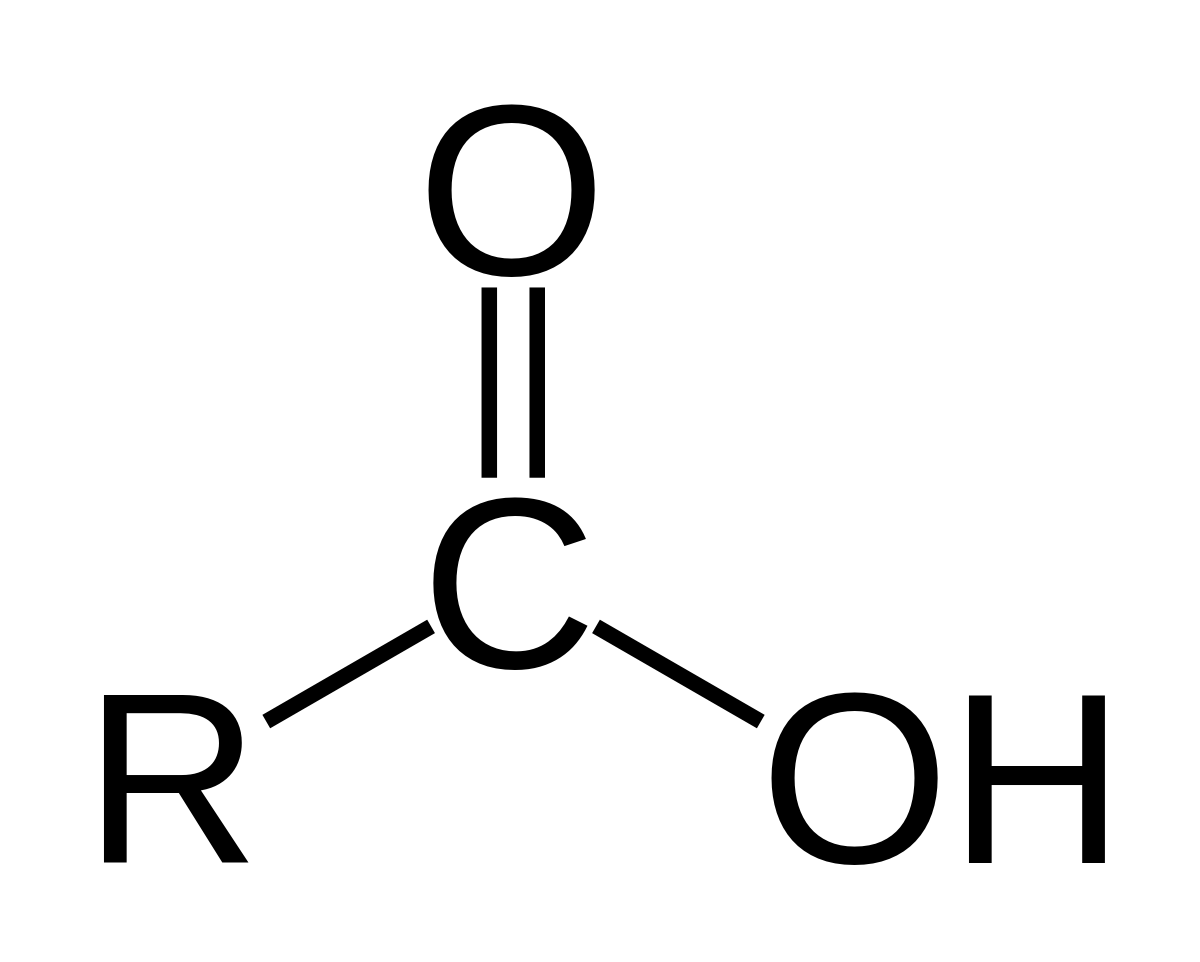
Carboxyl Group
Structure: O=C-OH; -COOH
Properties:
Polar
Acidic (donates H+ ions)
Hydrophilic
Found in:
Carboxylic acids (e.g., acetic acid)
Amino Group
Structure: -NH2
Properties:
Polar
Basic (accepts H+ ions)
Hydrophilic
Found in:
Amines (e.g., glycine)
Sulfhydryl Group
Structure: -SH (also written as HS-)
Properties:
Polar
Hydrophilic
Role:
Involved in forming disulfide bridges in proteins
Example: Cysteine
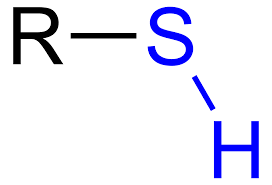
Methyl Group
Structure: -CH3
Properties:
Nonpolar
Hydrophobic
Example: 5-Methyl cytidine
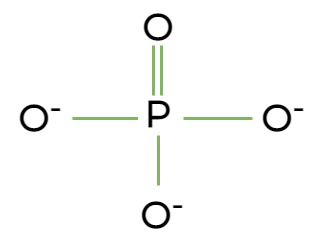
Phosphate Group
Structure: Contains negatively charged phosphate.
Reacts with water to release energy.
Important in molecules like ATP (adenosine triphosphate).
Charged
Polar
Hydrophilic
Adenosine Triphosphate (ATP)
ATP is a pivotal organic molecule that provides energy for cellular activities.
Emphasizes the importance of understanding ATP in biological processes.
Hormones and Functional Groups
Hormones provide males and females with unique secondary sexual characteristics.
Similar base structure but differ in functional groups, which determine their properties.
Drugs and Functional Groups
Examples of opioids:
Codeine
Morphine
Heroin
Oxycodone
Fentanyl (synthetic, 100x more potent than morphine)
Naloxone (used for reversing overdoses)
Chemical Reactions
Overview of chemical reactions: convert reactants into products.
Central to creating biological molecules in cells.
In-Class Question 1
Which functional group is polar and acts as a base?
A) Sulfhydryl
B) Carboxyl
C) Amino
D) Carbonyl
E) Hydroxyl
In-Class Question 2
Identify the missing functional group in the provided molecule:
A) Hydroxyl
B) Carbonyl
C) Carboxyl
D) Amino

In-Class Question 3
Identify the functional groups present in given molecules, excluding methyl groups.
In-Class Question 4
Analyze Atenolol, a common beta blocker used for high blood pressure.
Chemical Shorthand
Atenolol illustration:
Kekulé structure: All atoms and bonds drawn out.
Bond-line structure: Simplified, showing only essential bonds.
Vocabulary
Key terms to know:
Carbon
Phosphate group
Organic compounds
Chemical reaction
Hydrocarbon
Reactant
Isomers
Product
Functional group
Hydroxyl group
Carbonyl group
Carboxyl group
Amino group
Sulfhydryl group
Methyl group
 Knowt
Knowt