Mandatory Experiments
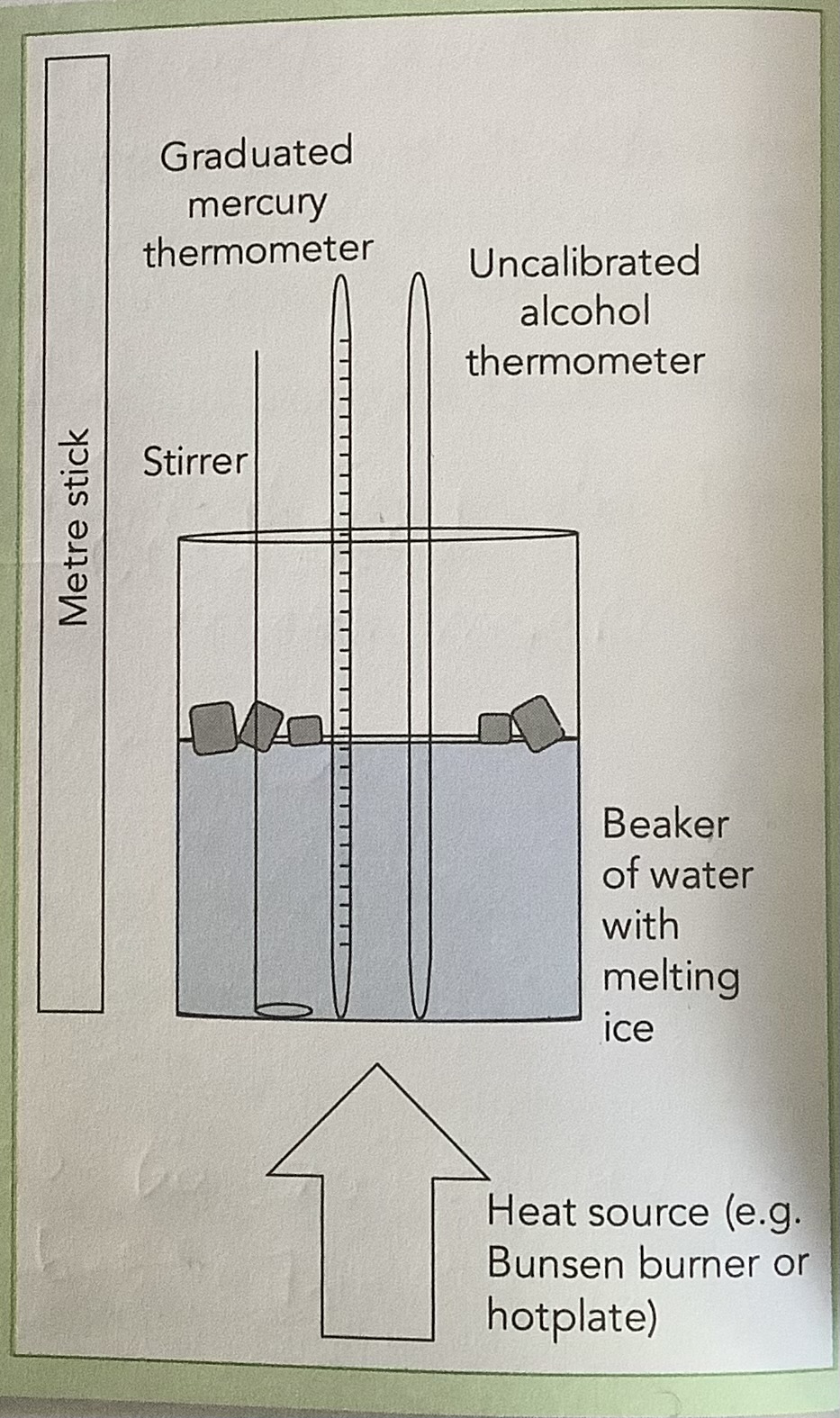
Plotting the Calibration Curve of a Thermometer using a Laboratory Alcohol Thermometer as Standard
Procedure:
Add ice to 50ml of water in a beaker with a graduated thermometer and an uncalibrated thermometer.
Read the temperature on the graduated thermometer and record it in a table.
Measure the length of the column of alcohol and record it in the table.
Put the beaker over a heat source and heat it gently.
Record the temperature and length of the column of alcohol every 10oC
Plot the graph of the length of the column of alcohol against temperature.
To use the alcohol thermometer to read temperature, measure the length of the column of alcohol and find the corresponding temperature using the calibration curve.
Accuracy:
Parallax error when reading the temperature.
Zero error when the temperature has not reached zero.
Diagram:
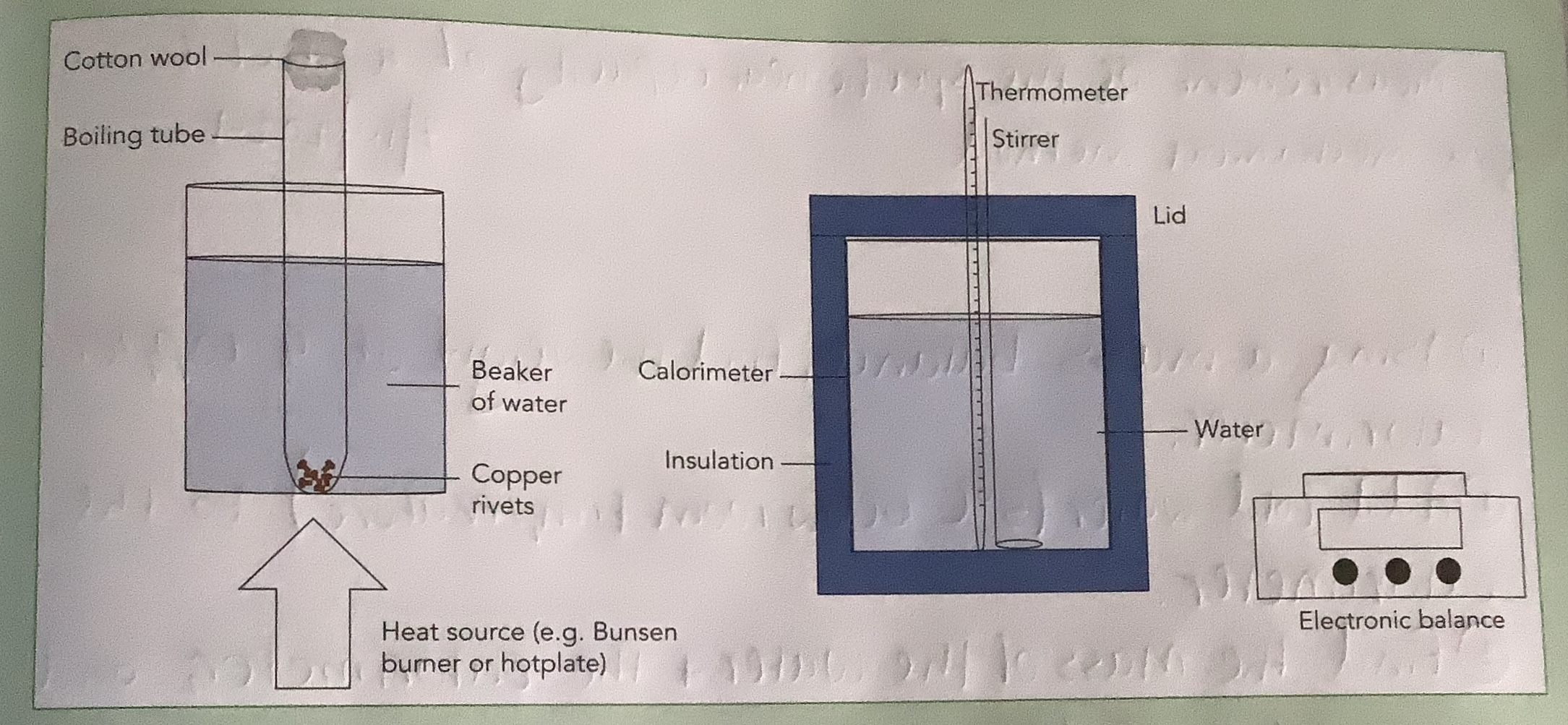
Measurement of the Specific Heat Capacity of a Metal by a Mechanical Method
Procedure:
Using a mass balance, find the weight of a copper calorimeter.
Add cold water (5oC below room temperature) to the calorimeter.
Find the mass of the water and calorimeter and convert it into kg.
Find the mass of the water by subtracting the mass of the calorimeter from the total mass.
Find the inital temperature of the water and copper by using a thermometer.
Find the mass of the copper rivets using a mass balance and convert it to kg.
Put the copper rivets into a boiling tube into a beaker of water and heat it to 100oC.
Add copper rivets to the calorimeter.
Record the final temperature of the water using a thermometer.
Calculate the specific heat capacity of the copper riverts, assuming no heat was lost to the surroundings.
Accuracy:
Make sure not to splash water when adding the rivets.
Use lid on calorimeter to ensure no heat is lost to surroundings.
Diagram:
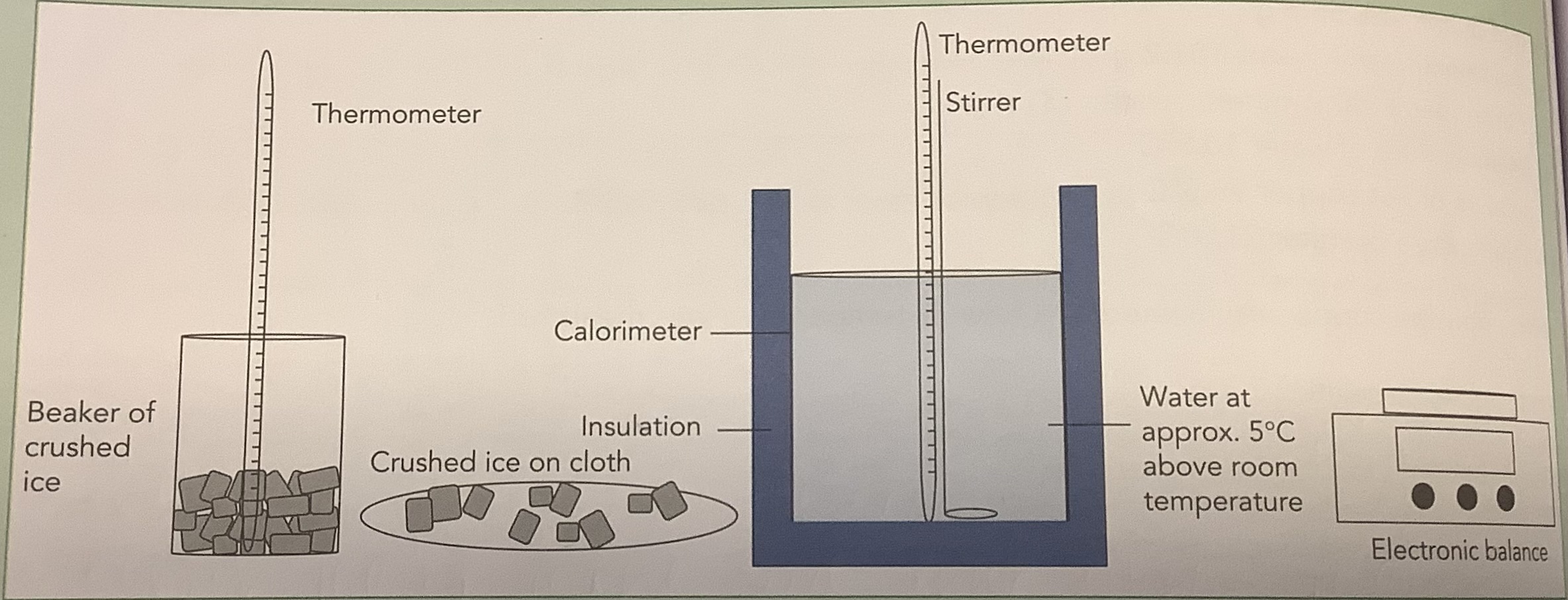
Measurement of the Specific Latent Heat of Fusion of Ice
Procedure:
Crush the ice, place it in a beaker and leave it for a few minutes until it is noticeably melted.
Take the temperature of the ice, and ensure it is at 0oC
Find the mass of the empty calorimeter using an electronic balance.
Heat some water to about 30oC in a beaker using the bunsen.
Fill the calorimeter about ¾ of the way with the hot water.
Find the combined mass of the calorimeter and the water, and find the mass of the water by subtracting the mass of the calorimeter from the combined mass.
Note the value of room temperature, and stir the warm water until the temperature is 5oC above room temperaure.
Place the calorimeter into a container of insulating material and record the value of the final temperature.
Dry the crushed ice thoroughly with the cloth.
Add the dried ice into the water until it is 5oC below room temperature.
Stir to evenly distribute the ice and record the temperature.
Find the combined mass of the calorimeter, water, and melted ice, and find the value of the ice by subtracting the mass of the calorimeter and water from the combined mass.
Use the information collected to solve for the specific latent heat of fusion of ice.
Accuracy:
Avoid spalshing the water when adding the ice as it may change the mass of the water.
Parallax error when reading the temperature.
Zero error if you are unable to get the ice to 0oc
Diagram:
Measurement of the Specific Latent Heat of Vaporisation of Steam
Procedure:
Find the mass of the calorimeter
Fill the calorimeter with cold water and find the mass
Set up a round bottomed flask and connect the steam trap
Brink flask to boil
Adjust flame so steam is emerging at a steady rate
Record temperature of cold water in calorimeter
Dry exit tube from steam trap with cloth
Place exit tube deep into cold water
Leave tube in water until a 12oC temperature change is noted
Remove the tube and turn off bunsen burner
Stir the water and record the highest temperature
Record the mass of the calorimeter and water and steam
Accuracy:
Dry steam tap to prevent excess water entering calorimeter
Ensure calorimeter is insulated to prevent heat loss to surroundings
Parallax error when reading temperature
Use thermometer graduated in steps of 0.1oC to get accurate temperature reading.
Diagram:
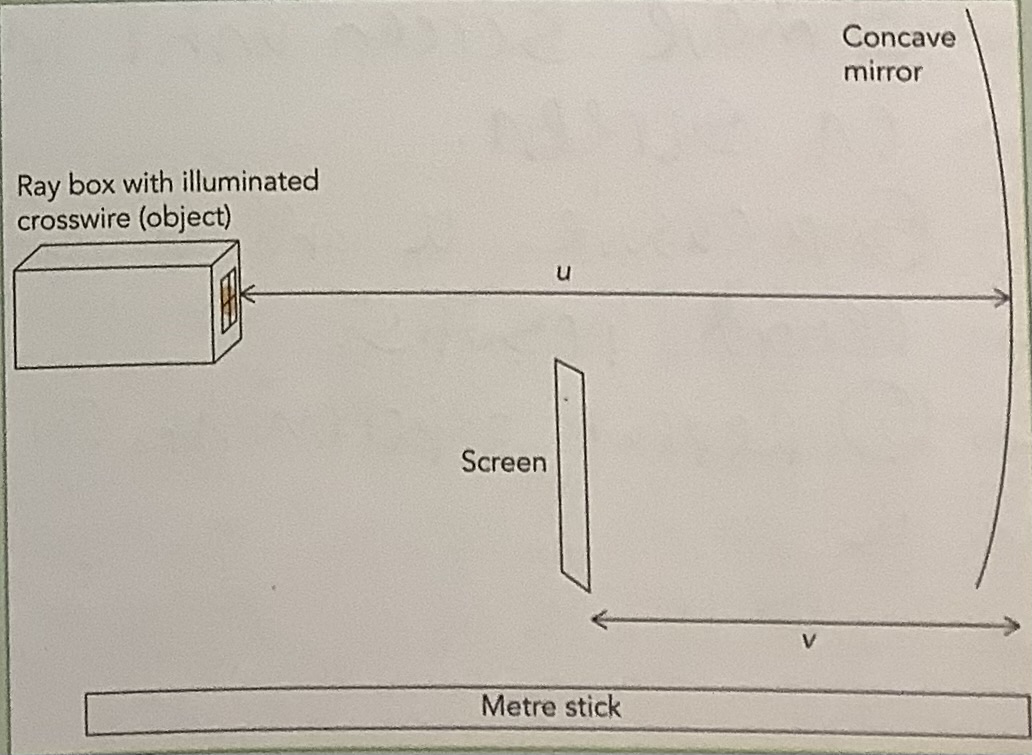
Measurement of the Focal Length of a Concave Mirror
Procedure:
Find the approximate focal length of the mirror by focusing the image of a distant object on a screen and measuring the distance between the mirror and the screen using a metre stick.
Set up the apparatus as shown in the diagram with the object positioned outside the approximate focal length.
Move the screen until the image of the crosswire is in sharp focus on the screen.
Measure u and v using a metre stick and record your results.
Repeat the experiment for different values of u.
Using your table of results, find an average value of f.
Accuracy:
Ensure that the image is in sharp focus before measuring v.
Avoid parallax error when using the metre stick.
Take measurements from the back of the mirror.
Take measurements from the centre (pole) of the mirror.
Ensure that both the screen and the mirror are vertical when taking measurements.
Find an approximate value of the focal length. This is to avoid placing the object inside the focal length when carrying out the experiment. If the object is placed inside the focal length, a virtual image will be formed and this cannot be located on a screen.
Diagram: