Bonding!
\n
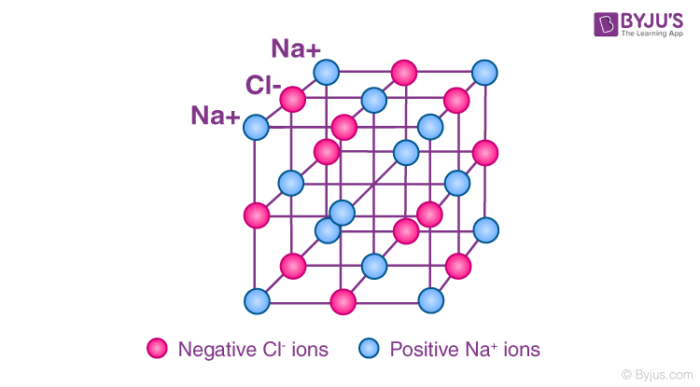
Ionic Compounds:
Ionic compounds consist of a metal and a nonmetal joined together by attractions between positive and negatively charged ions. They always have regular structures. They're giant structures because they contain millions of atoms joined together and the electrostatic attractions between the positive ions and the negative ions are very strong.
\n
It takes a lot of energy to break up these very strong bonds and so ionic compounds have very high melting and boiling points. Most ionic substances dissolve in water because water molecules are able to separate the positive and negative ions from each other and still hold them together in a solution. However when an ionic compound is in solution it changes its properties in a solid the ions. These are going to be positive, negative etc. In a solid, all of the ions are fixed in place so the particles can't move around. However if an ionic compound is in liquid form either it's been melted so it's molten or it's in a solution otherwise known as aqueous. Although the particles are close together the particles are able to move around and this gives us it a key property that's unusual and that is that it conducts electricity.
\n
Ionic compounds conduct electricity when they're in liquid form but not when they're in solid form because the ions are able to move around and carry charge.
\n
 \n
\n
\n
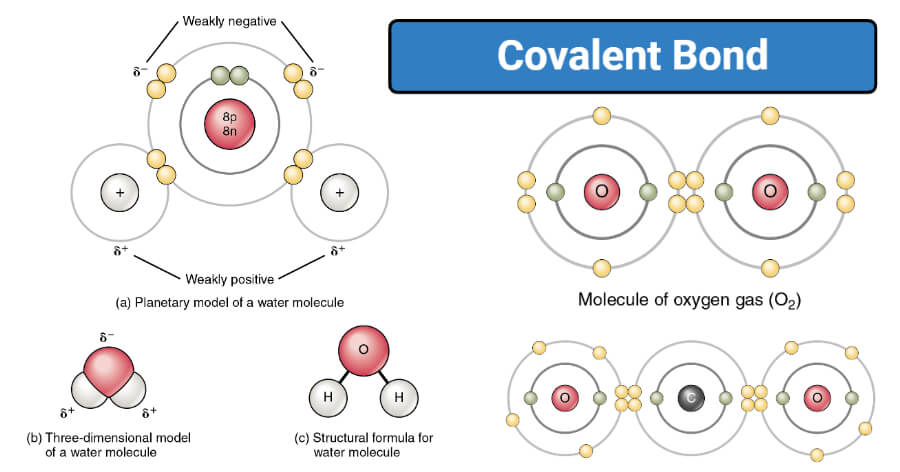
Covalent Compounds:
Small molecules have covalent bonding the bond between the atoms that's holding it together is very very strong. This covalent bond the attraction between the electrons that are shared between the atoms and the positive charges of the nucleus of the atoms. However small molecules in a substance have very weak forces that hold the molecules together so there's strong attractions inside the molecule but the attractions between the molecules are very weak.
\n
This means that it's easy to break the molecules apart from one another and that means small molecules otherwise known as simple covalent molecules have very low melting points this means they're usually gases or liquids at room temperature. Simple covalent molecules also do not conduct electricity because all of the electrons are used up in the covalent bond and there are no free electrons that can move around or carry charge. Remember when a substance like this is melted it's the weak forces between the molecules that break not the strong bonds inside the molecules.
\n
 \n
\n
\n
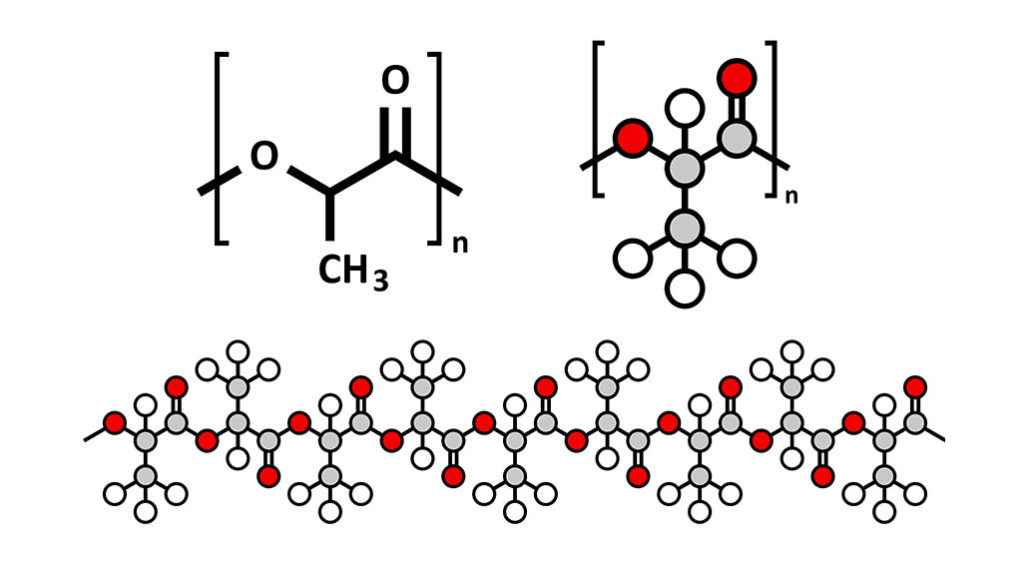
Polymers:
A polymer is a long chain of small molecules that have been joined together in a polymer structure. Each molecule is one of these long chains and they're joined together to the nearby chains with weak intermolecular forces the weak forces that were holding the molecules together. These simple covalent molecules the smaller the molecule is the weaker the forces between the molecules but the longer the molecule is the more forces there are between the molecules and therefore it takes more energy to break the larger molecules apart from one another.
\n
Because polymers are so long they have more weak forces between the molecules therefore they have higher melting points and because of this polymers are usually solid so that's one type of covalent molecule the simple covalent molecule otherwise known as molecular covalent.
\n
There are also giant covalent structures such as diamond and graphite. Remember these are both forms of carbon and another example is silicon dioxide the common name is quartz or sand and these are all substances which have a giant covalent structure just like we had with ionic substances giant means that it contains millions of atoms joined together covalent of course means the type of bonding that holds the atoms together the shared pair of electrons unlike simple molecules giant covalent structures always have very high melting points they also have high boiling points too and this is because it needs a lot of energy to overcome the strong attraction between the atoms in the covalent bonds. In general giant covalent structures such as diamond and silicon dioxide do not conduct electricity but graphite is an exception and it does conduct electricity.
\n
 \n
\n
\n
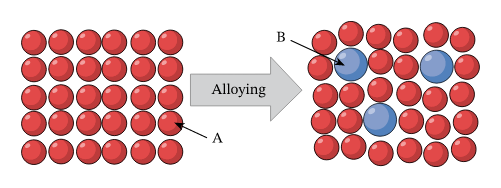
Metals:
Metals are also giant structures meaning they have millions of atoms joined together and the atoms are held together by strong metallic bonds. This means that in general they have high melting and boiling points because it takes a lot of energy to break apart the metallic bonds but metals are malleable and ductile meaning that they can be bent and shaped and they can be drawn into wires and this is because the layers of metal ions can slide past each other and thereforemove around.
\n
However because of this a lot of pure metals are too soft to be used in things like saucepans hammers buildings cars, etc, so we can adapt their properties by making them into alloys which are mixtures of metals and these alloys help to harden and strengthen the pure metal by disrupting the even layers in the metal structure making it harder for the layers to slide past each other. Pure metals and alloys are both good conductors of heat and electricity because the thermal and electrical energy can be carried by the sea of delocalized electrons that holds the atoms together.
\n
 \n
\n
\n
Summary:
In summary we have three types of bonding in ionic compounds a metal and a nonmetal formed positive and negative ions and the ions are trapped as strongly together because of their opposite charges. Covalent substances are where nonmetals joined together by sharing a pair of electrons between the two nuclei in metallic bonding. This contains metals only in a regular arrangement called a lattice and in between the layers of metal ions there are delocalized electrons that can move and flow through the substance.
\n
There's two types of structure giant and then simple molecular so ionic covalent and metallic all have giant structures but covalent is the only one that has the simple molecules all of the giant structures have high melting points. It's the simple molecular ones that have low melting points this is because in the giant structures the forces that hold the atoms together are very very strong and therefore a lot of energy is needed to overcome them in simple molecules the melting and boiling points are low because although the bonds holding the atoms together are very strong. The forces that hold the molecules together in the substance are very weak so the weak forces between the molecules are quite easy to break.
\n
Ionic compounds dissolve in water and when they dissolve in water or if they melt but to form a liquid they will conduct electricity but they won't conduct electricity as a solid because the ions aren't able to move around. Giant covalent substances will not dissolve in water and simple and simple molecules will sometimes dissolve in water.
\n
Covalent structures in general do not conduct electricity except for the one exception of graphite which does conduct electricity because graphite contains delocalized electrons similar to metals. All metals conduct electricity because they have delocalized electrons in their structure that are able to flow through and carry the charge of the electric current.
\n
\n \n \n  \n
\n