Atomic Structure (AQA)
Atoms
Atoms have a nucleus at the center, which contains protons and neutrons. Electrons move around the nucleus in shells.
The number of these tiny particles in an atom can be determined using its atomic number and mass number.
Early Theories About Atoms
Scientific ideas about atoms have changed over time as new discoveries were made.
John Dalton (1803) – Suggested that all matter is made of tiny, solid spheres called atoms, which cannot be divided.
J. J. Thomson (1897) – Discovered the electron and proposed the plum pudding model, where the atom is a ball of positive charge with negative electrons spread throughout, like fruit in a pudding.
Ernest Rutherford (1909) – Tested the plum pudding model by firing alpha particles at thin gold foil. Most particles passed through, but some bounced off. This led to a new idea: the nuclear model of the atom.
Rutherford’s Nuclear Model
Rutherford’s experiment showed that:
The nucleus is at the center of the atom.
The nucleus is positively charged.
Most of the atoms are empty.
This model replaced the plum pudding idea and became the foundation for our modern understanding of atoms.
Development of Atomic Models
Scientists have changed their understanding of atoms over time as they discovered new evidence.
Niels Bohr (1913) – Electron Shells
Suggested that electrons move in fixed orbits (shells) around the nucleus.
Energy levels are at specific distances, and electrons can jump between them.
Discovery of Protons (1917)
Rutherford confirmed the existence of protons, positively charged particles in the nucleus.
James Chadwick (1932) – Discovery of Neutrons
Found evidence for neutrons, neutral particles that add mass but have no charge.
Explained why atomic mass is greater than the total mass of protons and electrons.
Modern Atomic Model
Atoms consist of a nucleus (protons and neutrons) and surrounding electron clouds.
Quantum mechanics suggests that electrons do not follow fixed orbits but exist in probability regions.
The model helps explain chemical reactions, bonding, and properties of elements.
Structure of an Atom
An atom has a small nucleus at its center.
Electrons move around the nucleus in different layers called shells.
The nucleus is much smaller than the whole atom:
The size of an atom is about 0.1 nanometers (1 × 10⁻¹⁰ m).
The nucleus is about 1 × 10⁻¹⁴ m, which is thousands of times smaller than the atom.
For comparison:
A bacterium is about 1 × 10⁻⁶ m.
A human hair is about 1 × 10⁻⁴ m.
Subatomic particles
The nuclei of all atoms contain subatomic particles called protons. The nuclei of most atoms also contain neutrons.
The masses of subatomic particles are very tiny. Instead of writing their actual masses in kilograms, we often use their relative masses.
The relative mass of a proton is 1, and a particle with a relative mass smaller than 1 has less mass.
Subatomic particle: Proton
Relative mass: 1
Relative charge: +1
Subatomic particle: Neutron
Relative mass: 1
Relative charge: 0
Subatomic particle: Electron
Relative mass: Very small
Relative charge: -1
The mass of an electron is very small compared to a proton or a neutron. Since the nucleus contains protons and neutrons, most of the mass of an atom is concentrated in its nucleus.
Protons and electrons have electrical charges that are equal and opposite.
Key fact: Remember that protons are positive, and neutrons are neutral.
Atomic number and mass number
Atomic number
The atomic number is the number of protons in an atom of an element.
All atoms of the same element have the same number of protons.
Different elements have different numbers of protons.
An atom has the same number of protons and electrons. Since protons have a positive charge and electrons have a negative charge, the total charge of an atom is neutral.
For example, sodium (Na) has an atomic number of 11, meaning every sodium atom has 11 protons and 11 electrons. This results in 11 positive charges and 11 negative charges, making the atom neutral.
Mass number
The mass number of an atom is the total number of protons and neutrons in its nucleus.
Atoms of different elements usually have different mass numbers, but some can be the same.
For example, both argon and calcium can have a mass number of 40.
Calculating numbers of subatomic particles
An element’s atomic symbol can show its mass number (top) and atomic number (bottom).
To determine the number of subatomic particles in an atom:
Number of protons = atomic number
Number of electrons = atomic number
Number of neutrons = mass number - atomic number
Isotopes
Atoms of the same element always have the same number of protons, but they can have different numbers of neutrons. Atoms with the same number of protons but different numbers of neutrons are called isotopes.
Characteristics of isotopes:
Same atomic number
Different mass numbers
Chemical properties of isotopes:
All isotopes of an element have the same chemical properties.
This is because chemical properties depend on the number of electrons, and all isotopes of an element have the same number of electrons.
Relative atomic mass (Ar)
The relative atomic mass of an element is the weighted average of the masses of its isotopes, considering their abundance (how common they are).
Relative atomic masses are listed in the periodic table with the symbol Ar.
Key points:
Mass numbers are always whole numbers.
Relative atomic masses are often not whole numbers because they are an average.
Example: Chlorine — the relative atomic mass of chlorine is 35.5, not a whole number, because chlorine has two isotopes:
Chlorine-35 and Chlorine-37
Calculating Relative Atomic Mass
The carbon-12 atom is used as the standard for comparing the masses of other atoms. The relative atomic mass (Ar) of an element is the average mass of its atoms, measured relative to 1/12th the mass of a carbon-12 atom.
How is relative atomic mass calculated?
It is based on:
The mass numbers of an element’s isotopes
The abundance (percentage) of each isotope
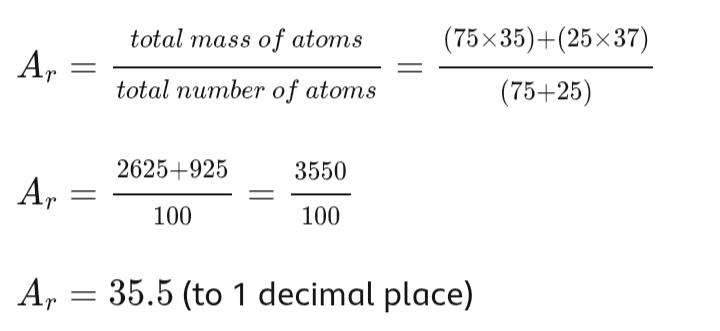
Example: Chlorine
Chlorine exists as two isotopes:
Chlorine-35 (75% abundance)
Chlorine-37 (25% abundance)
For every 100 chlorine atoms:
75 atoms have a mass number of 35
25 atoms have a mass number of 37
To calculate the relative atomic mass, Ar, of chlorine:
Key Point:
The relative atomic mass of chlorine is 35.5.
The value is closer to 35 because chlorine-35 is more abundant than chlorine-37.