Chapter 5: The Second Law of Thermodynamics
three statements of the second law establish the theoretical performance for systems undergoing cycles while interacting with thermal reservoirs
power cycle efficiency
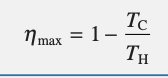
refrigeration cycle
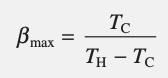
heat pump cycle
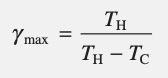
Key Terms and Concepts
Clausius statement - states that it is impossible for any system to operate in such a way that the sole result would be an energy transfer by heat from a cooler to a hotter body
thermal reservoir - a special kind of system that always remains at a constant temperature even though energy is added or removed by heat transfer
Kelvin-Planck statement - states that it is impossible for any system to operate in a thermodynamic cycle and deliver a net amount of energy by work to its surroundings while receiving energy by heat transfer from a single thermal reservoir
entropy statement - it is impossible for any system to operate in a way that entropy is destroyed
irreversible process - when the system and all parts of its surroundings cannot be exactly restored to their respective initial states after the process has occurred
reversible - if both the system and surroundings can be retured to their initial states
irreversibilities
heat transfer through a finite temperature difference
unrestrained expansion of a gas or liquid to a lower pressure
spontaneous chemical reaction
spontaneous mixing of matter at different compositions or states
friction-sliding friction as well as friction in the flow of fluids
electric current flow through a resistance
magnetization or polarization with hysteresis
inelastic deformation