Lecture 11 Actin World
Page 2: Learning Objectives
Characterize actin filament structure and composition.
Formation and dynamics of thin filaments: Understanding how they are formed in cells and the roles of associated motor proteins.
Actin-associated proteins: List and distinguish their functions.
Role of thin filaments: Distribution and diverse functions in cellular contexts.
Muscle tissue structure and function: Analyzing actin's contribution to muscle activity.
Cellular movement mechanisms: Outlining how actin facilitates cellular motility.
Actin Filaments
Major Protein Component: Actin is integral to all cells, located at cell junctions and throughout cellular structures.
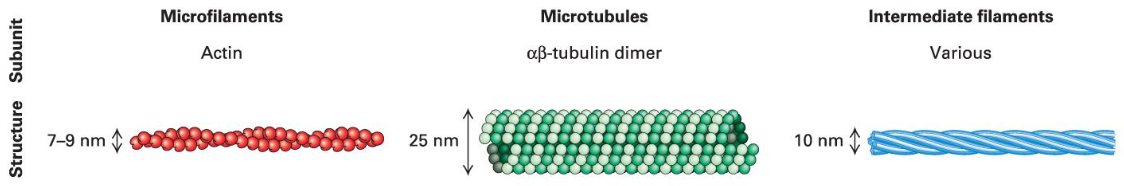
Cytoskeletal Filaments
Types of cytoskeletal filaments:
Actin: Also known as the basic unit of thin filaments/microfilaments, vital for a multitude of functions. (side roads)
Intermediate filaments: Provide tensile strength against stress.
Microtubules (thick filaments): Serve various functions, acting as pathways for intracellular transport. (major highways)
Actin Dynamics
Dynamic structure: Actin constitutes 10% of muscle cell protein and 1-5% in non-muscle cells.
Filament formation and disassembly: Actin filaments are continuously formed and disassembled, tailored to cellular needs and environmental stimuli.
Actin genes: Humans have six actin genes that contribute to the diversity of actin functions.
Functions of Actin
Diverse roles: Actin is involved in cellular structure, movement, phagocytosis (“eating”), and cell division.
Three types of actins:
α-actin: Found in muscle fibers.
β-actin: Present in the leading edge of cells.
γ-actin: Associated with stress fibers.
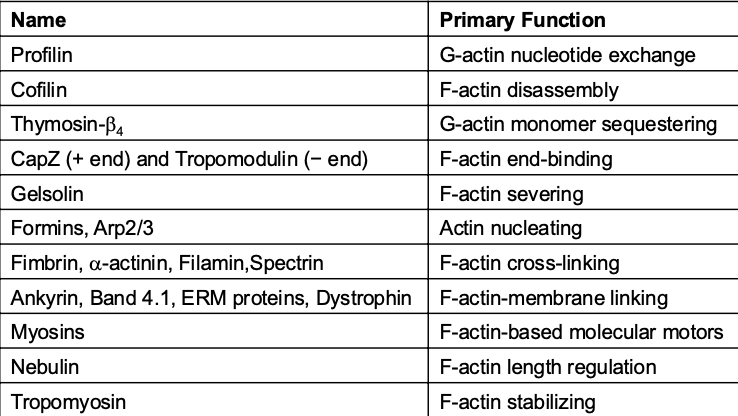
Actin-Binding Proteins
Table of Proteins: Overview of actin-binding proteins and their functions, e.g., Profilin (nucleotide exchange), Cofilin (disassembly), and various capping and cross-linking proteins.
Functionality: Proteins that can promote actin polymerization, stabilize filaments, or sever actin structures. Proteins associated with actin can modify it by nucleating, stabilizing, serving, capping, etc.
Actin Monomers
Actin exists as G-actin: Globular actin binds ATP and Mg2+.
Polymerization: Forms filamentous actin (F-actin) with 2 helical structures wrapped around one another. .
Polarization of Actin Filaments
Polar characteristics: Myosin S1 regions coat F-actin, creating a polarized structure with distinct ends for filament dynamics.
form arrow like structures:
(-) pointed end: end has cleft exposed to the solution
(+) barbed end: has the cleft contacting other subunits
Filament Dynamics
End addition preference: G-actin adds more readily to the (+) end of the filament compared to the (-) end, influencing overall growth.
to form filaments G-actin-ATP units are added to both ends.
(+0) end addition is almost 10x greater than the (-) end
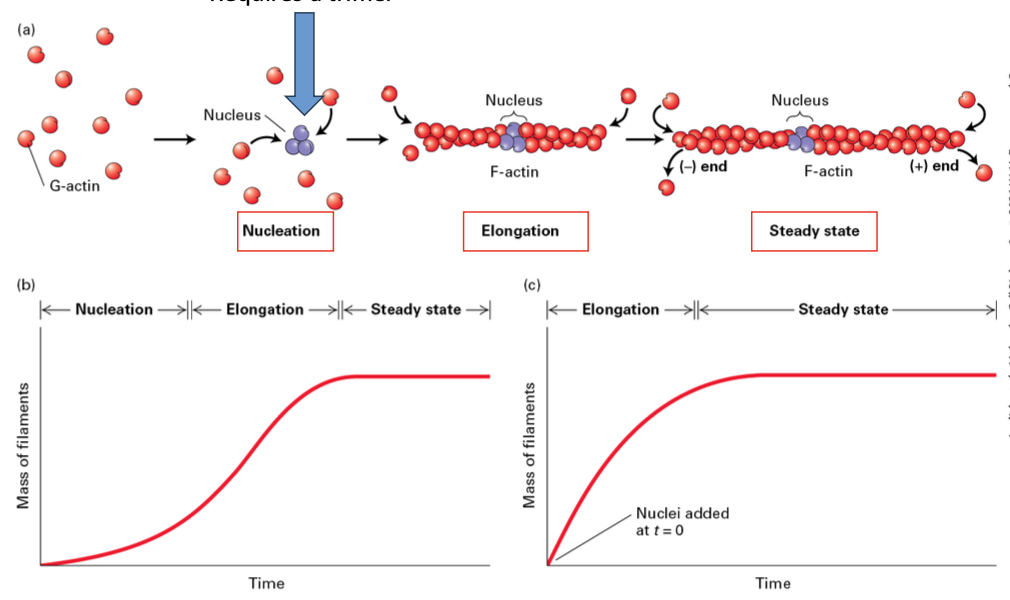
Actin Filaments need a Critical Concentration
Requirement for filament growth: Actin filaments need a critical concentration of G-actin-ATP. Each different Cc which much lower [Cc] required in the (+) end.
ATP is hydrolyzed quickly when actin monomers are added creating 3 distinct regions.
Treadmilling is a dynamic equilibrium process in filament assembly and disassembly. Seen at steady state the (-) end loses monomers while new ones are added to the (+) end
Steps of Actin Filament Formation
Nucleation
Elongation
Steady State
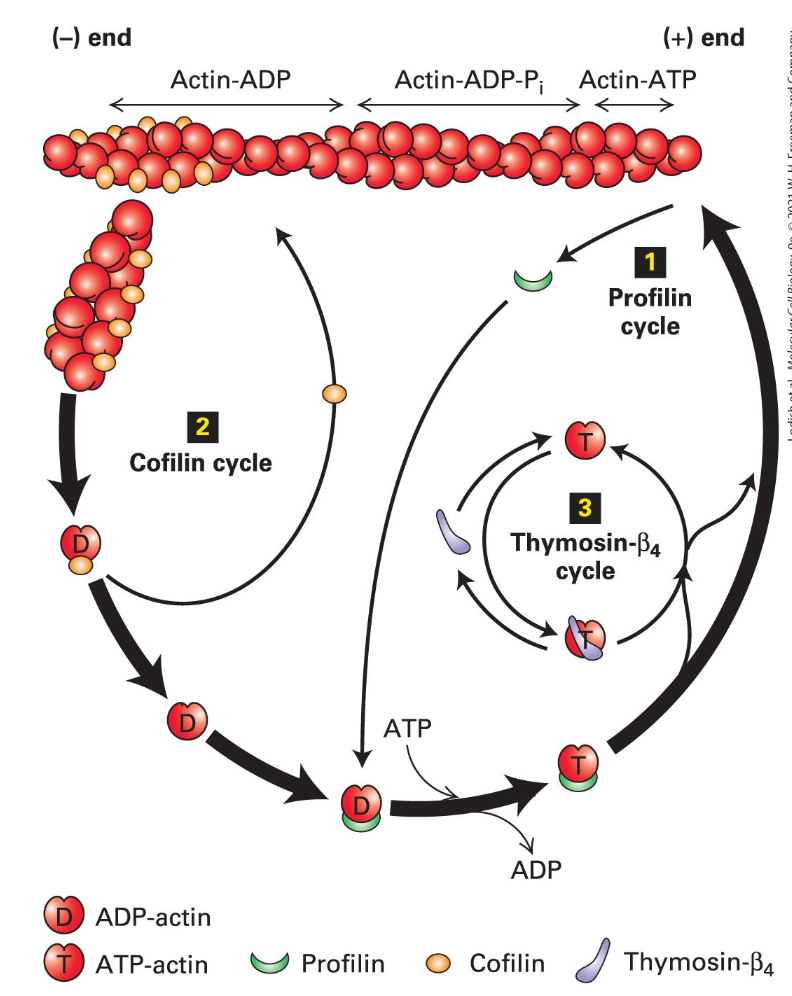
Actin Recycling
Profilin's role: Enhances actin recycling and promotes the binding of ATP-actin at the (+) end, contributing to dynamic filament reassembly.
Profilin binds G-actin lost from filaments and opens the cleft enhancing loss of ADP
Profilin-ATP-actin complex binds the (+) end
Cofilin binds 2 ADP-actin towards the (-) end, causes a twist and a break
Generates more (-) ends for disassembly
Thysomin - B4 binds free ATP-actin to keep it all from assembling needless into filaments
Forms dynamic equilibrium with the free subunits
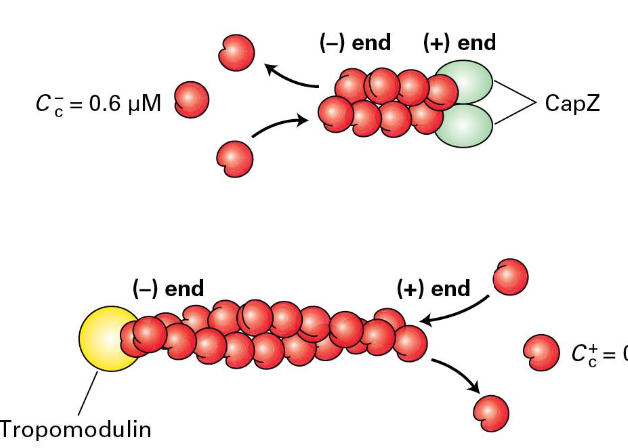
Capping Proteins
To regulate the formation and length of filaments/dynamics
CapZ binds the (+) end
Formed from two related proteins
Cells regulate CapZ ability to stop growth
Phosphatidylinositol 4,5- bisphosphate (PIP2)
Regulator proteins
Tropomodulin binds the (-) end and can module filament assembly and disassembly
Mostly found where actin needs to be stabilized
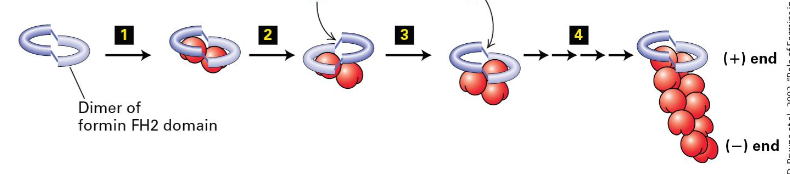
Actin-Nucleating Proteins
2 major classes of actin-nucleating proteins help in the assembly of F-actin cells.
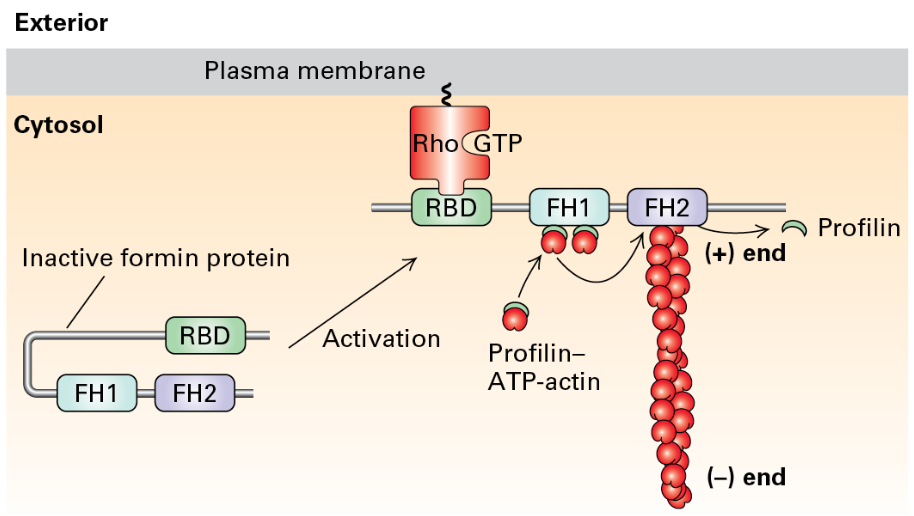
Formin proteins: Facilitate long filament assembly.
Arp2/3 complex: Promotes branched filament networks.
Formins Homology 1, 2 Domains in Formins
FH1 domains bind profilin to increase ATP-actin
FH2 domains form a ring that rocks back and forth promoting addition of ATP actin to the (+) end
Regulatory Signaling
Signal pathways: Actin nucleation and filament formation can be controlled through receptor-mediated signaling pathways, leading to the activation of formin proteins.
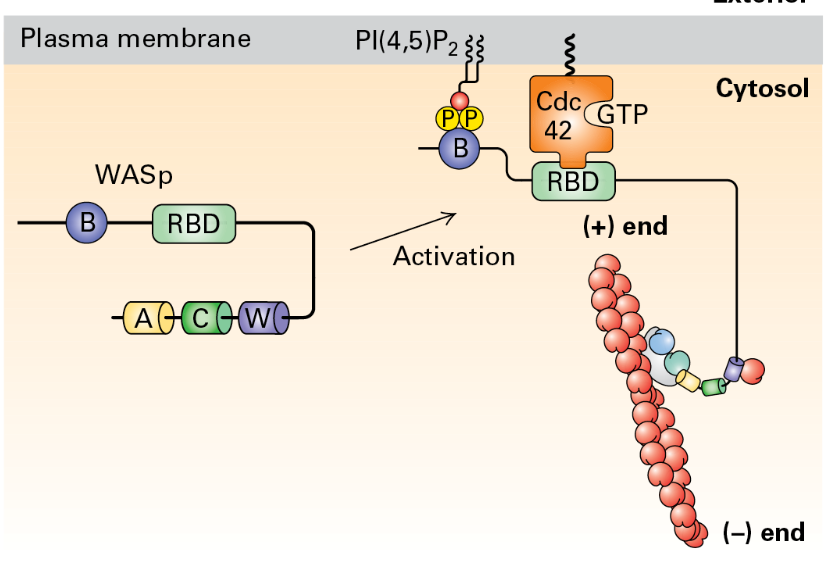
ARP2/3 and NPFs
Complex function: The ARP2/3 complex promotes nucleation and creation of branched F-actin structures, activated by nucleation-promoting factors. This binds to existing F-actin and promotes branch formation.
WASp Activation
WASp's (Wiskott-Aldrich syndrome) role: Serves as a nucleation-promoting factor by activating signaling cascades that stimulate Arp2/3 near cellular membranes.
Listeria Monocytogenes
Overview of Gram-positive bacteria: Causes listeriosis with significant health risks, particularly for pregnant women and immunocompromised individuals.
ActA protein mimcs an NPF and activated and recruits Arp2/3. Actin polymerization propels the bacteria through the cell.
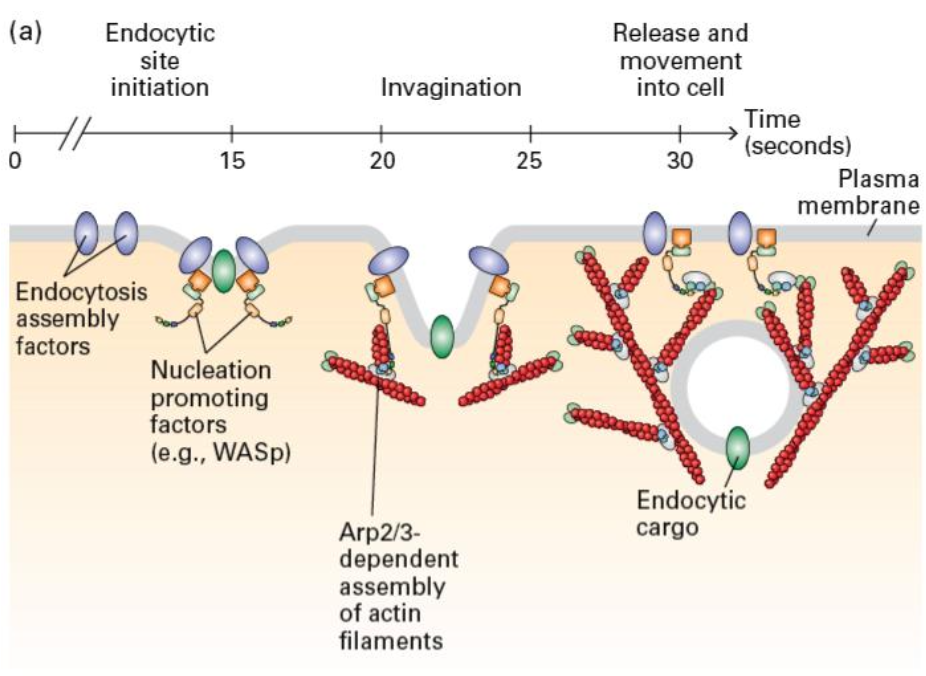
Actin Role in Membrane Dynamics
Membrane mechanisms: Actin polymerization generates force and movement and aids in endocytosis, with contributions from signaling factors that assist in membrane dynamics.
Bursts of Arp 2/3 activation recruits other factors that can help power this energetically unfavorable membrane dynamic
Phagocytosis
Immune function: Describes how actin aids immune cells in engulfing pathogens through receptor-mediated signaling and network formation.
Opsonized pathogen are immobilized by the cell and receptor mediate signaling activates local Arp2/3 activation and network formation. creates a contractile network that leads to isolation of the pathogen and subsequent destruction.
Cross-Linking Proteins
Structural diversity: Actin cross-linking proteins mold filaments into varied structures, essential for functions such as sound perception in stereocilia.
Erythrocyte Structure
Stress handling: Actin networks are crucial for erythrocyte integrity, maintaining its shape and structure under stress. Actin membrane is anchored by actin associated proteins such as a/B spectrin. Actin gives cell shape and structure.
Actin Filaments and Muscle
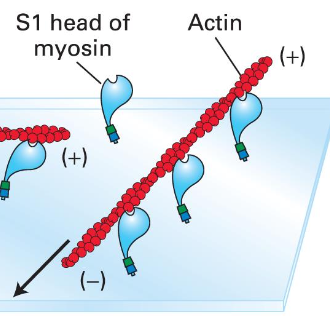
Myosin II was the first discovered motor protein, integral in muscle cell function by converting chemical energy to mechanical work. All family motor proteins can bind to actin.
Immobilized ATPase containing mysoin S1 subunits will move actin filaments under experimental conditions. 40 myosin genes in the human genome.
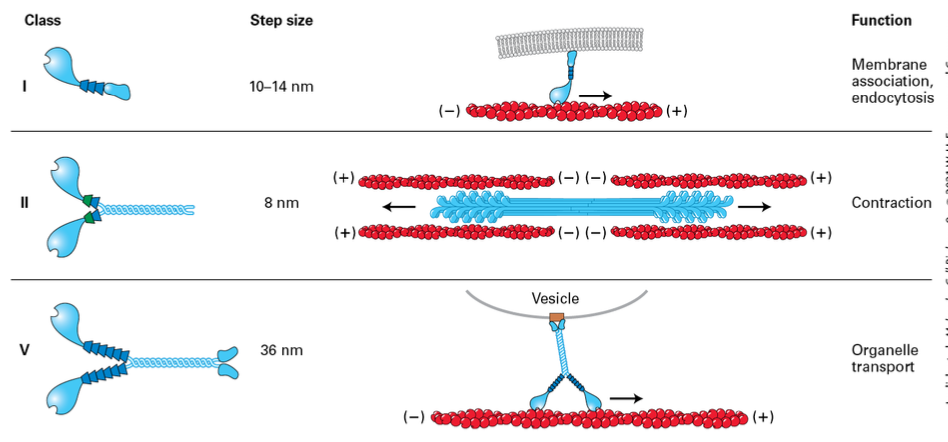
Myosin Classes
Myosin I – only one with asingle head domain and it is associated with the membrane
Myosin II – mostly in muscle (thick fibers) but also non-muscle in less organized fashion, used for contraction
Myosin V – motor protein to hall things towards the membrane
Myosin VI – only member that moves towards the negative end, moving things away from the membrane
Page 29: Myosin-Actin Interaction
Power stroke dynamics: Myosin-ADP binds to F-actin. ATP binds releasing myosin from F-actin Hydrolysis cocks the head domain. Head domain binds actin. Release of Pi moves it back into its original position. In muscle 10% of the myosin is contacting f-actin when activated.
Myosin Steps
Step size of myosin head is relative to the length of the neck domain. (longer legs = bigger step),
Myosin II moves about 8nm
Myosin V takes longer 72 nm steps, hauling cargo 36nm ata. step
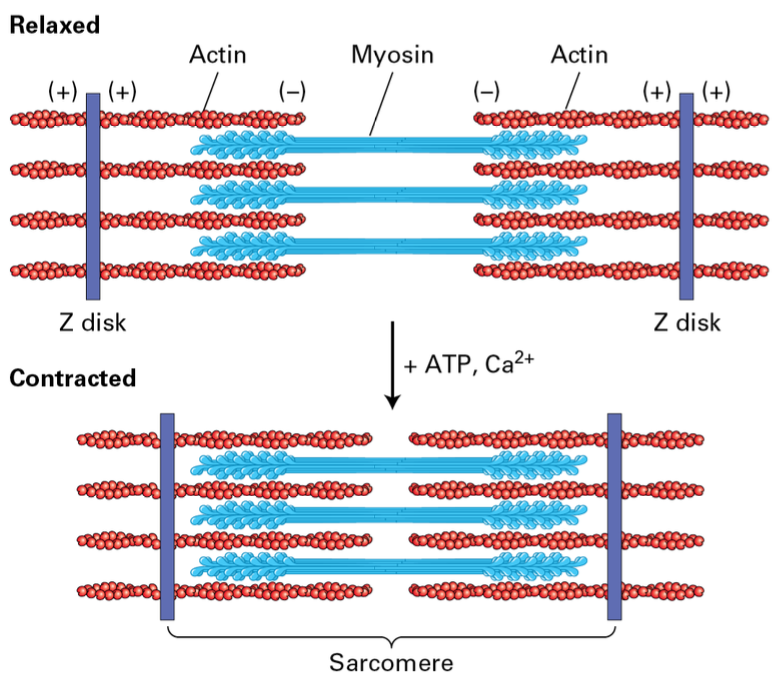
Muscle Fiber Composition
muscles —> muscle fibers (multinucleated muscle cells) —> myofibril structures composed of sacromere
Z disk structures flank: Myosin II thick filaments alternating with F-actin thin filaments attached to the Z disk
Thick filaments are bipolar with heads out toward Z disks. F-actin is attached to Z-disk. During contraction the mysoin II moves towards the (+) shortening the distance between the (-) end of F-actin.
Controlled by ATP availability and Ca2+ influx
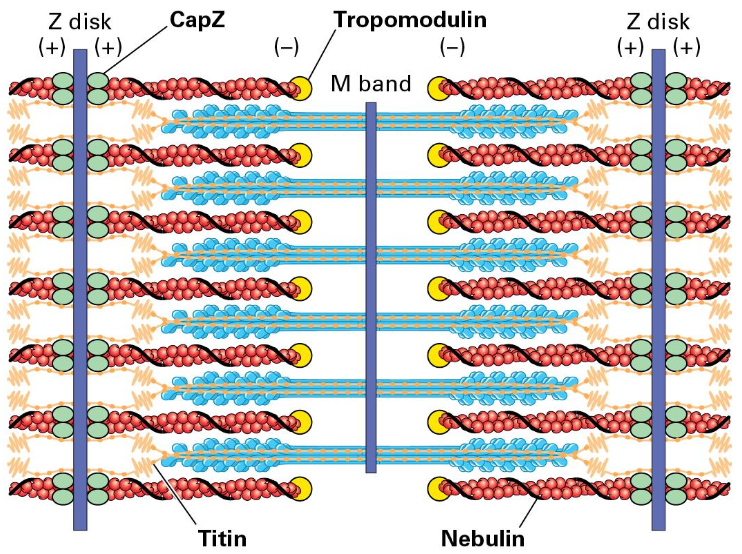
Accessory Proteins in Muscle
Structural support: CapZ, Tropomodulin, Titin, and Nebulin play crucial roles in maintaining muscle structure and function.
CapZ helps bind F-actin (+) and Tropomodulin (-)
a giant elastic titin protein binds Z disk and extends toward the M band on either side (holds the thick filaments in place)
Giant nebulin protein wraps around the F-actin
Cytokinesis
Role of actin: Highlighting the involvement of actin-myosin in cytokinesis, forming contractile rings that assist in cell division.
Sarcoplasmic reticulum (SR) holds a high store of Ca2+ for skeletal muscle regulation
Neural impulse travels down the transverse tubules and opens voltage- gated Ca2+ channels in the SR
The release of Ca2+ allows muscle contraction by binding Troponin
Troponin shifts Tropomyosin
Actin-Mysoin II is involved in Cytokinesis. A contractile ring forms after chromosomal partition and eventually pinches off.
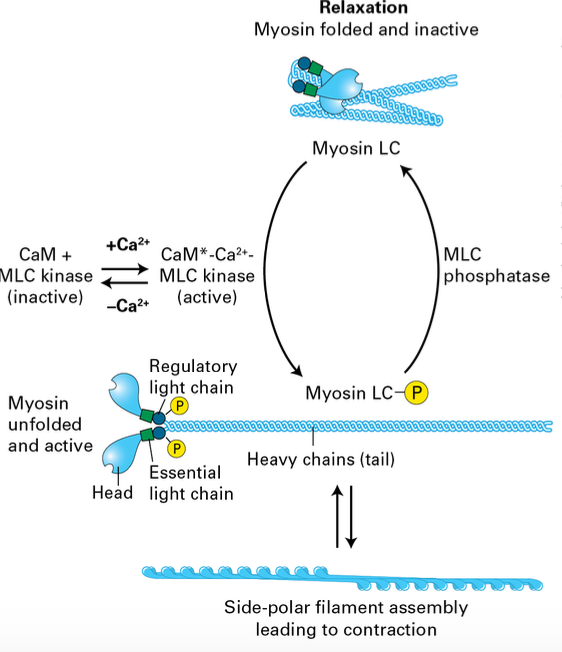
Smooth Muscle Contraction
Regulatory mechanisms: Smooth muscle contractions are controlled by signaling pathways rather than calcium levels alone.
Myosin is inactive during relaxation
Phosphorylation activates it forming filaments that can induce contraction in smooth muscle.
Dephosphorylation relaxes it again.