Acids, bases and salts
Key vocabulary:
Aqueous: dissolved in water
Acid: source of hydrogen ions (H⁺) in solution
Hydrogen ion: hydrogen atom that has lost an electron (H⁺)
Proton: another name for a hydrogen ion (H⁺)
Alkali: source of hydroxide ions (OH⁻) in solution
Solubility: how well a substance dissolves
Soluble: does dissolve
Insoluble: does not dissolve
Dissociation: breaking apart into ions
Ionisation: forming ions by gaining or losing electrons
Neutralisation: reaction of acid with base/alkali to form a salt
Acids:
Acids release hydrogen ions (H⁺) in solution and act as proton donors.
They can be strong or weak depending on how much they ionise in water.
Strong acids: Almost fully ionised in water, producing many H⁺ ions.
Examples: hydrochloric acid (HCl), sulphuric acid (H₂SO₄), nitric acid (HNO₃).
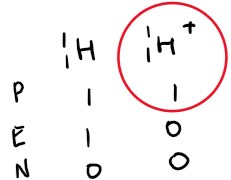
Equation to show how strong acids ionise completely in water to form many H+ ions:
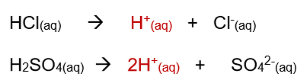
Weak acids are those that are partially ionised in water, forming few H+ ions
e.g ethanoic acid,CH3COOH(aq) and carbonic acid, H2CO3(aq),
Equation to show how ethanoic acid ionises partially in water to form few H+ ions:
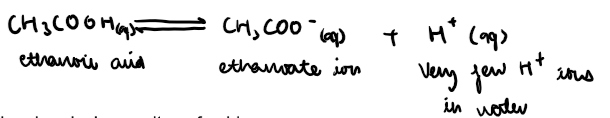
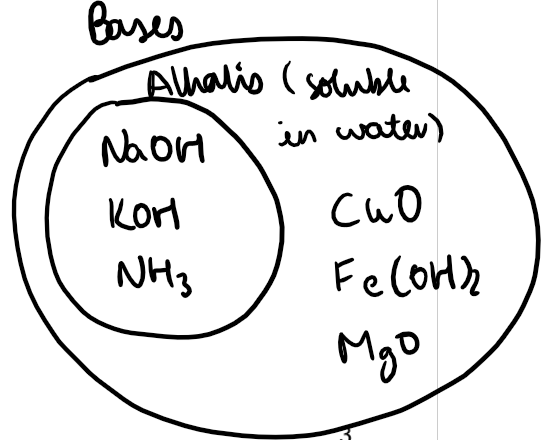
Bases:
Proton acceptors and opposites of acids.
Include metal oxides, hydroxides, and ammonia.
Neutralise acids to form a salt and water.
Examples:
Sodium hydroxide (NaOH)
Ammonia solution (NH₃)
Magnesium oxide (MgO)
Copper(II) oxide (CuO)
Iron(II) hydroxide (Fe(OH)₂)
Alkalis are bases that dissolve in water.
Alkaline solutions contain hydroxide ions (OH⁻).
Alkalis:
Strong alkalis: Fully dissociate in water, forming many OH⁻ ions.
Examples: sodium hydroxide (NaOH), potassium hydroxide (KOH)
Equation (for NaOH):
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Weak alkalis: Partially ionise in water, forming fewer OH⁻ ions.
Example: ammonia solution (NH₃)
Equation:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The more H⁺(aq) ions, the stronger the acidity and the lower the pH.
The more OH⁻(aq) ions, the stronger the alkalinity and the higher the pH.
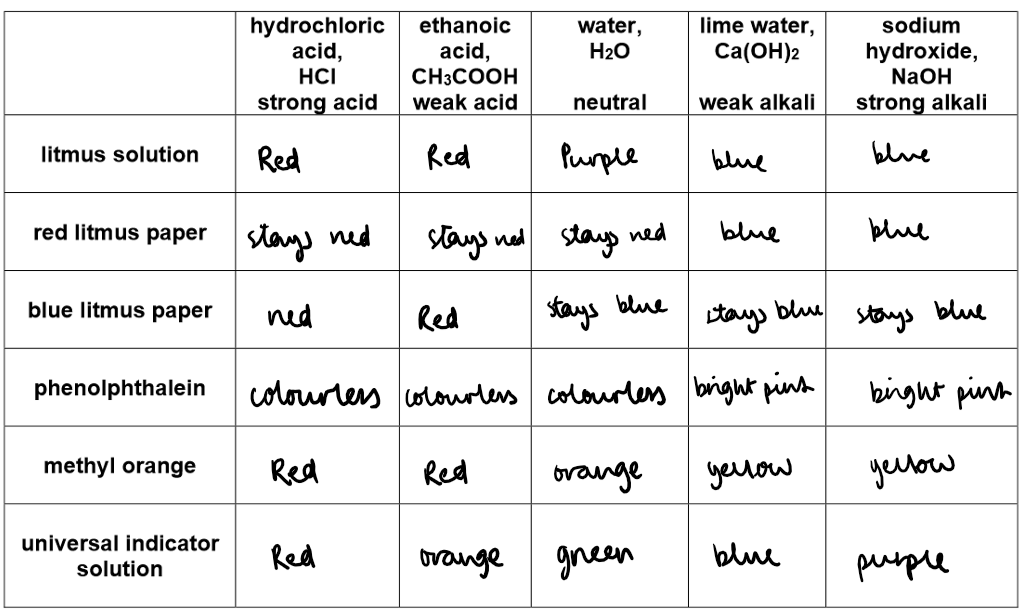
Indicators show if a solution is acidic, alkaline, or neutral by changing colour.
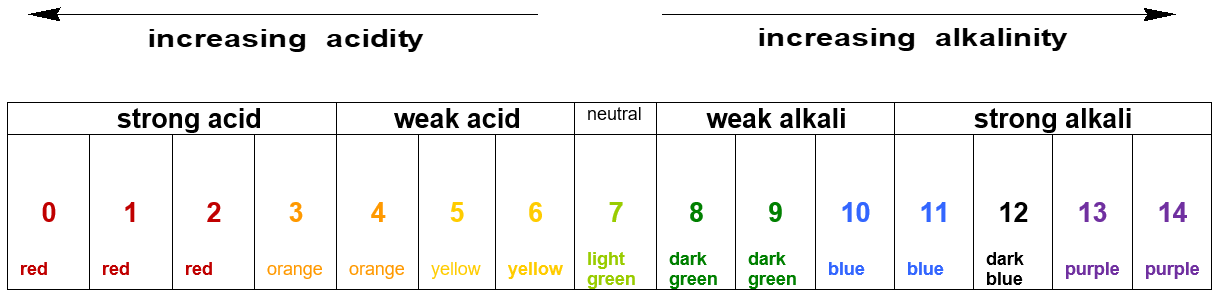
pH Scale:
The pH scale ranges from 0 to 14.
It measures how acidic or alkaline a solution is.
pH < 7: Acidic
pH = 7: Neutral
pH > 7: Alkaline
pH values as shown by the different colours with Universal Indicator solution
pH Values:
pH values are not always whole numbers (e.g., 2.4 or 5.8).
pH > 7: Alkaline
pH = 7: Neutral
pH < 7: Acidic
Universal Indicator is used to find the approximate pH of a solution – it has a range of colours corresponding to different pH values, indicating whether a solution is acidic, alkaline, or neutral.
Reactions of Acids:
Safety:
Wear goggles and a lab coat.
Ensure stools are tucked under the desks.
Procedure:
In this practical, investigate the reactions of acids.
Make a note of your observations and consider the inferences based on these reactions.

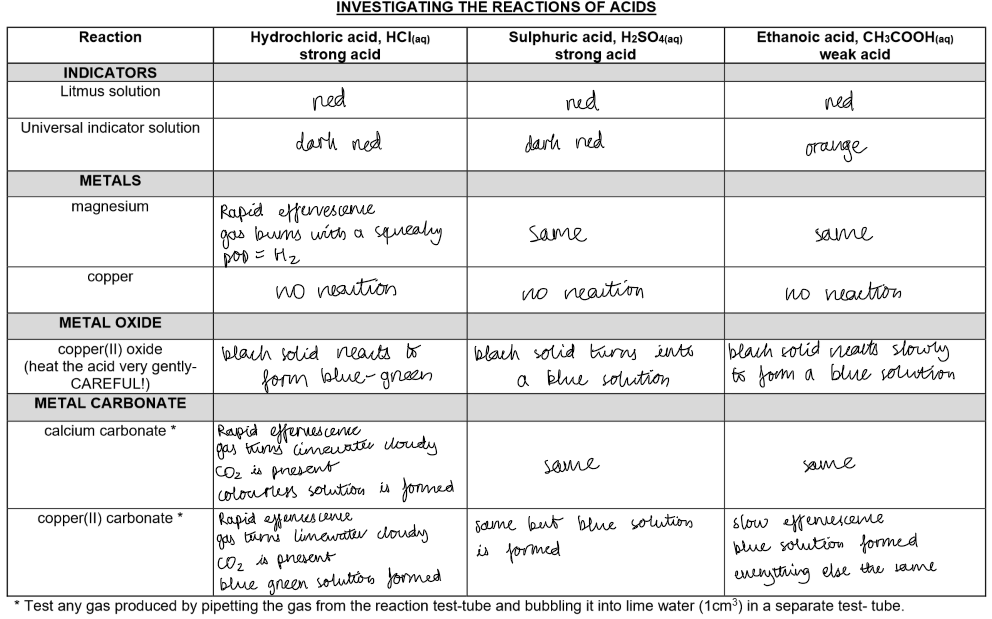
Properties of Acids:
pH:
Acids have a pH < 7, indicating they are acidic.Reaction with Metals:
Reactive metal + acid → salt + hydrogen
Example:
Zinc (Zn) + Hydrochloric acid (HCl) → Zinc chloride (ZnCl₂) + Hydrogen (H₂)
Salt Formation:
A salt is formed when an acid reacts with a metal or a base, replacing the hydrogen ions of the acid with metal or ammonium ions.Neutralisation with different acids gives different salts:
Hydrochloric acid → Chlorides
Sulphuric acid → Sulfates
Nitric acid → Nitrates
Ethanoic acid → Ethanoates
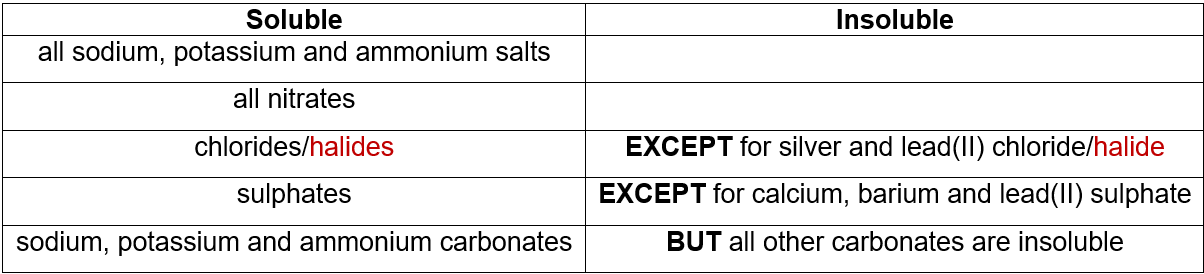
Solubility Rules:
State symbols can be added to chemical equations.
Acids are aqueous (aq) in water.
Water is a liquid (l).
Metals are solids (s).
Bases are solids (s) unless soluble in water, in which case they are alkalis (aq).
The state symbol for the salt depends on the metal or compound involved.
Solubility Rules
Salts can be made using different methods according to their solubility. Learn the following table:
Examples of Metals Reacting with Acids:
Magnesium + Sulphuric Acid
Word equation: Magnesium + Sulphuric acid → Magnesium sulfate + Hydrogen
Chemical equation: Mg(s) + H₂SO₄(aq) → MgSO₄(aq) + H₂(g)
Ionic equation: Mg(s) + 2H⁺(aq) → Mg²⁺(aq) + H₂(g)
Zinc + Hydrochloric Acid
Word equation: Zinc + Hydrochloric acid → Zinc chloride + Hydrogen
Chemical equation: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Ionic equation: Zn(s) + 2H⁺(aq) → Zn²⁺(aq) + H₂(g)
Observations:
Bubbles of hydrogen gas are released (effervescence).
The metal dissolves, and a salt solution forms.
Note:
Reactions with highly reactive metals (K, Na, Li) are too violent and dangerous.
Acid Reaction Equations: IMPORTANT!!!
Acid + Metal → Salt + Hydrogen
Acid + Base → Salt + Water
Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
Acid + Metal Hydrogen Carbonate → Salt + Water + Carbon Dioxide
Acid + Ammonia → Ammonium Salt
Common Acids:
Hydrochloric acid: HCl
Sulphuric acid: H₂SO₄
Nitric acid: HNO₃
Ethanoic acid: CH₃COOH
3) Reaction with Base or Alkali – Neutralisation:
Base or Alkali + Acid → Salt + Water
Examples:
Word equation: Copper(II) oxide + Hydrochloric acid → Copper(II) chloride + Water
Chemical equation: CuO(s) + 2HCl(aq) → CuCl₂(aq) + H₂O(l)
Ionic equation: CuO(s) + 2H⁺(aq) → Cu²⁺(aq) + H₂O(l)
Word equation: Potassium hydroxide + Sulphuric acid → Potassium sulfate + Water
Chemical equation: 2KOH(aq) + H₂SO₄(aq) → K₂SO₄(aq) + 2H₂O(l)
Ionic equation: 2OH⁻(aq) + 2H⁺(aq) → 2H₂O(l)
4) Reaction with Metal Carbonates and Metal Hydrogen Carbonates:
Metal carbonate + Acid → Salt + Water + Carbon dioxide
Metal hydrogen carbonate + Acid → Salt + Water + Carbon dioxide
Examples:
Word equation: Magnesium carbonate + Sulphuric acid → Magnesium sulfate + Water + Carbon dioxide
Chemical equation: MgCO₃(s) + H₂SO₄(aq) → MgSO₄(aq) + H₂O(l) + CO₂(g)
Ionic equation: MgCO₃(s) + 2H⁺(aq) → Mg²⁺(aq) + H₂O(l) + CO₂(g)
Word equation: Sodium hydrogen carbonate + Hydrochloric acid → Sodium chloride + Water + Carbon dioxide
Chemical equation: NaHCO₃(aq) + HCl(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
Ionic equation: NaHCO₃(aq) + H⁺(aq) → Na⁺(aq) + H₂O(l) + CO₂(g)
Observations:
Bubbles of carbon dioxide gas are produced(effervescence).
The metal carbonate or hydrogen carbonate dissolves.
5) Reaction with Ammonia Solution to Produce Ammonium Salts:
Ammonia + Hydrochloric acid → Ammonium chloride
Equation: NH₃(aq) + HCl(aq) → NH₄Cl(aq)
Ammonia + Sulphuric acid → Ammonium sulfate
Equation: 2NH₃(aq) + H₂SO₄(aq) → (NH₄)₂SO₄(aq)
Ammonia + Nitric acid → Ammonium nitrate
Equation: NH₃(aq) + HNO₃(aq) → NH₄NO₃(aq)