Protozoan Cell Biology and Disease
Visceral parasitic infections are caused by amoebae eg Entamoeba, ciliates eg Balantidium, and the coccidia flagellates and apicomplexa. Example flagellates are Giardia and Trichomonas, whereas example apicomplexa are cryptosporidium and toxoplasma.
Entamoeba histolytica is a pathogenic protist, transmitted to humans through contaminated water and food. The disease it causes is known as Amoebiasis. E. hisolytica is anaerobic and the infective form produces cysts which are resistant to gastric acids in the stomach. Excystation produces 8 trophozoites per cyst in the small intestine. Infection can be asymptomatic or lead to diarrhoea and/or dysentery. If untreated, the amoebae may invade the liver and in 10% of cases, the lungs and brain. It is diagnosed by cysts in stool observed by microscopy and is treated with azoles, in particular metronidazole. Paromomycin or iodoquinol is used for luminal amoebiasis.
Iodoquinol = iron chelator and is not taken up by intestinal cells.
How does metronidazole work? The iron-sulphur protein pyruvate: ferredoxin oxidoreductase (POR), obtained by horizontal gene transfer, reduces metronidazole to its active form, producing covalent protein and DNA adducts → death of parasite. Reduction of metronidazole nitro group gives nitroso-containing intermediates which can covalently bind DNA disrupting helical structure, inducing strand breaks and inhibiting nucleic acid synthesis → cell death.
Entamoeba histolytica life cycle:
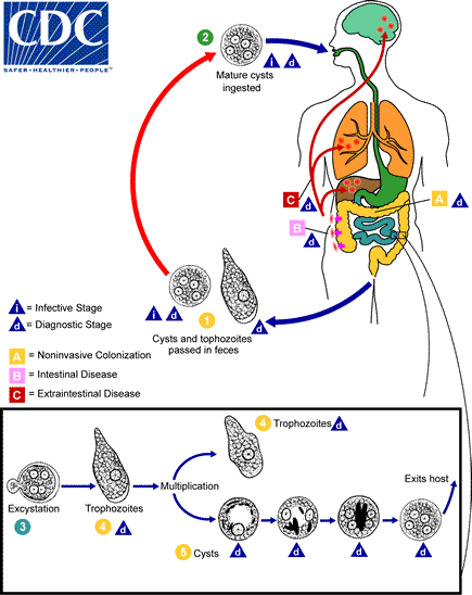 Mature cysts ingested. Non invasive colonisation of the small intestine occurs. Intestinal disease occurs in the small and large intestine when excystation occurs and trophozoites dorm which multiply. Extraintestinal disease is caused when left untreated. Cysts and trophozoites are passed in the faeces and the cycle continues.
Mature cysts ingested. Non invasive colonisation of the small intestine occurs. Intestinal disease occurs in the small and large intestine when excystation occurs and trophozoites dorm which multiply. Extraintestinal disease is caused when left untreated. Cysts and trophozoites are passed in the faeces and the cycle continues.
Balantidium coli is a ciliated, intestinal parasite of both humans and swine. it’s transmitted to humans via faecally contaminated water and infection is caused by cysts. The symptoms are similar to amoebiasis. Diagnosis occurs from stools and colon tissue and it is treated with tetracycline, metronidazole and iodoquinol. It’s thought that tetracycline may inhibit protein synthesis by binding mitochondrial ribosomes.
B. coli life cycle: The cyst is the infectious stage and is acquired by ingestion of contaminated food/water. Cysts form trophozoites which multiply and some invade the wall of the colon. Cysts and trophozoites are excreted.
Giardia intestinalis is a flagellated anaerobic parasite. It has mitosomes, which are mitochondrial remnant organelles not reliant on the electron transport chain. G. intestinalis produces highly resistant cysts and is the cause of giardiasis, a common waterborne disease. Symptoms of giardiasis are explosive foul-smelling diarrhoea, intestinal cramps, nausea, weight loss and malaise. However many individuals exhibit no symptoms and can act as carriers. Giardiasis is diagnosed through cysts and trophozoites found in faeces and is treated with metronidazole and tinidazole.
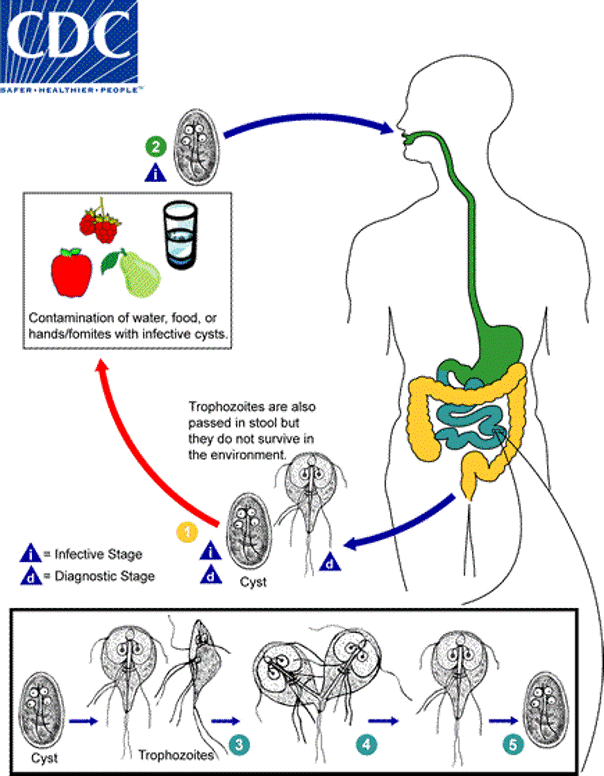
Life cycle:
Infective cysts are ingested and remain in the intestine. Cysts form trophozoites. Cysts and trophozoites are excreted but the trophozoites do not survive in the environment.
Trichomonas vaginalis is a flagellated anaerobic parasite transmitted by sexual intercourse. It can survive on moist surfaces so can be transmitted by toilet seats, sauna benches and towels. It’s mainly asymptomatic in mains but causes vaginal purulent discharge, itching and burning in women. Purulent = contains pus. Diagnosis occurs by microscopy and cell culture from patient secretions. Efficient treatment occurs with metronidazole.
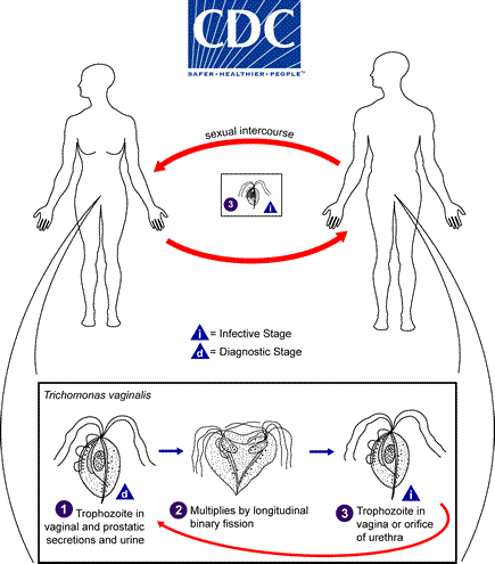
Life cycle of T. vaginalis
Trophozoites are found in the vaginal and prostatic secretions as well as urine. They multiply by longitudinal binary fission and colonise the vagina or orifice of the urethra of another person when the infected individual has sexual intercourse.
Cryptosporidium parvum is a protist of phylum Apicomplexa which lives as a parasite in warm-blooded animals and causes diarrhoea. It produces thick walled cells called oocysts, which are shed in the faeces of infected animals and transmitted in faecally contaminated water such as swimming pools. Oocysts are highly resistant to chlorine and filtration methods so sedimentation and filtration methods are most effective at removal.
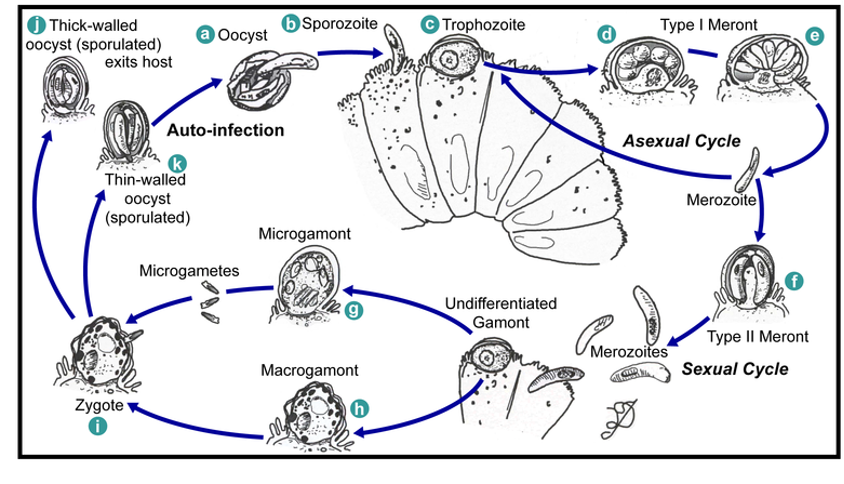
C. parvum has many unique features. The parasite develops just under the epithelial cell membrane, in an intracellular but extracytoplasmic position. The oocyst stages are fully formed when expelled from the host. C. parvum does not require sporulation meaning oocysts are immediately infective - this is known as auto-infection. Its diagnosis occurs from direct fluorescence antibody assay of a stool sample. The infection is self-limited but can be treated with nitazoxanide which interferes with anaerobic energy metabolism.
Events in the intestine of a human infected with C. parvum: sporozoites form inside oocysts which develop into trophozoites. An asexual cycle where of trophozoite → type 1 meront → merozoite occurs, or merozoite develops into type II meront which goes onto form merozoites in a sexual cycle. Undifferentiated gamonts form micro and macrogamonts, allowing zygotes to form which produce thin and thick walled oocysts. Thick walled oocysts exit by excretion and thin walled remain in the intestine.
 Toxoplasma gondii is an apicomplexan protist which is a parasite of warm blooded animals. Its life cycle is similar to Cryptosporidium parvum with the same intermediate stages. It produces oocysts which are shed in faeces of infected animals and transmitted by cats and undercooked meat. Toxoplasmosis is mainly asymptomatic but can damage the eyes, brain and other organs in immunocompromised individuals. Toxoplasma can cause birth defects eg hydrocephalus if a mother is infected while pregnant. Toxoplasma is treated with Sulphadiazine and Pyrimethamine.
Toxoplasma gondii is an apicomplexan protist which is a parasite of warm blooded animals. Its life cycle is similar to Cryptosporidium parvum with the same intermediate stages. It produces oocysts which are shed in faeces of infected animals and transmitted by cats and undercooked meat. Toxoplasmosis is mainly asymptomatic but can damage the eyes, brain and other organs in immunocompromised individuals. Toxoplasma can cause birth defects eg hydrocephalus if a mother is infected while pregnant. Toxoplasma is treated with Sulphadiazine and Pyrimethamine.
Blood and tissue parasitic infections include Naegleria and Acanthamoeba infection, and malaria.
Naegleria fowleri is a free-living amoeba in soil and water. Infection usually arises from swimming in warm, soil-contaminated water eg hot springs or lakes. It enters the human body via the nose and burrows directly into the brain, causing extensive haemorrhage and brain damage - meningoencephalitis. In most cases, N. fowleri is fatal. It’s diagnosed from the cerebrospinal fluid, and drug treatment is only effective if the infection is identified early. Azoles, amphotericin B, rifampicin and miltefosine are used.
There are many species of Acanthamoeba such as castellani, polyphaga and culburtsoni. They are found in fresh and salt water throughout the world. Infection occurs through contaminated contact lenses or contact lens cases. They can also infect broken skin causing cutaneous disease or spread to the brain via blood. They may also infect the mucosa then the brain in a similar way to Naegleria.
Eye disease = Acanthamoeba keratitis (AK)
Skin disease = cutaneous Amoebiasis
Brain disease = Granulomatous Amoebic Encephalitis (GAE)
Diagnosis of Acanthamoeba occurs by microscopic examination of tissue samples and it’s treated with azoles, amphotericin B, rifampicin and miltefosine. Acanthamoeba exist in two forms, the active trophozoite in favourable conditions and under stress such as drug pressure, lack of nutrients or lack of magnesium, it forms non-dividing, environmentally resistant cysts.
Acanthamoeba keratitis is a progressive, sight-threatening corneal disease for which the leading risk factor is contact lens wear. It’s the most common type of Acanthamoeba infection. Treatment isn’t always successful and corneal transplants are often required, however reactivation can occur in the transplanted cornea.
Malaria is caused by the apicomplexan parasite Plasmodium. It has a complex life cycle including Anopheles mosquitoes as vectors. Sporogenic cycle occurs in the mosquitoes. In humans, the exo-erythrocytic cycle occurs in the liver and the erythrocytic cycle occurs in the blood. Each year, around 350 million are infected worldwide and over 1 million die every year of malaria. It’s generally found in tropical and subtropical regions. However, malaria was endemic in the UK into the 20th century. Swamp drainage removed mosquito breeding grounds, eradicating malaria.
Sir Ronald Ross won the Nobel prize for the elucidation of mosquito-transmission of malaria.
Diagnosis of malaria requires identification of Plasmodium-infected erythrocytes in blood smears by microscopy. They may appear in the trophozoite ring stage, as gametocytes or as schizonts. Drugs are used both to prevent and treat malaria, eg chloroquine and mefloquine (Lariam), pyrimethamine, doxycycline and clindamycin. Malaria may recur years after primary infection. Several vaccines are currently in development and malaria can be controlled by draining swamps or eliminating mosquitoes.
5 species of Plasmodium are able to infect humans with malaria. P. malariae causes Malaria quartana which has 72 hour paroxysms. P. vivax and P. ovale cause Malaria tertiana which has 48 hour paroxysms. P. falciparum causes Malaria tropica which has irregular 48 hour paroxysms. P. knowlesi is limited to Southeast Asia and causes severe malaria. A paroxysm is a bout of fever.
Trypanosomatids (flashcard 65) cause blood and tissue parasitic infections such as Leishmaniasis, human African Trypanosomiasis aka sleeping sickness, and Chagas disease.
Leishmania is a flagellated protozoan which causes cutaneous, mucocutaneous or visceral leishmaniasis. It’s transmitted by sandfly bites and infects and grows in macrophages. Leishmaniasis is diagnosed by microscopy of a tissue specimen, PCR and serology.
Life cycle of Leishmania:
In sandflies: Sandfly takes a blood meal, ingesting macrophages infected with amastigotes. The amastigotes transform into promastigote stage in the midgut and divide then migrate to the proboscis. The infected sandfly bites another individual and the parasite enters the ‘human stage’.
In humans: promastigotes are phagocytosed by macrophages. The promastigotes transform into amastigotes inside macrophages. The amastigotes multiply in the cells of various tissues, including macrophages.
Leishmania mexicana causes cutaneous leishmaniasis and nodules and ulcers form on the skin. It’s treated with pentavalent antimonial compounds, amphotericin B and miltefosine. However, antimonial compounds are quite toxic to the human body and many patienrs died from taking this drug. Resistance has risen against it. Amphotericin was a drug designed to fight cancer but was found to be more effective against Leishmaniasis. However it is quite expensive.
Leishmania braziliensis causes mucocutaneous leishmaniasis. The parasite destroys the mucosa and cartilage of the mouth, nose and throat. Treated with pentavalent antimonial compounds, amphotericin B and miltefosine. Can be fatal if untreated.
Leishmania donovani causes visceral leishmaniasis (VL). The parasite travels to internal organs and causes damage to the liver, spleen and bone marrow. Recovery is possible but occasionally relapse occurs as it’s not possible to know if all the parasites have been killed, this is known as post-kala-azar dermal leishmanoid (PKDL). It’s treated with pentavalent antimonial compounds, amphotericin B and miltefosine. VD fatal if left untreated.
Trypanosoma is a relative of Leishmania. There are two subspecies of Trypanosoma brucei, known as T. brucei gambiense and T. brucei rhodesiense, the latter being the more severe form if infected. T. brucei causes African sleeping sickness - human African trypanosomiasis (HAT). 55 million are at risk of HAT. It’s transmitted by the bite of the tsetse fly and the parasite multiplies in the blood. During stage I of infection, the early stages, there is an intermittent fever.
Stage II sleeping sickness affects the neuronal system as the parasites cross the blood-brain barrier into the brain. This leads to inflammation of the brain tissue and necrosis. Diagnosed by spinal puncture and microscopy of cerebrospinal fluid. Requires hospitalisation. The meningoencephalitic stage is where the CNS is invaded, causing headaches, somnolence, abnormal behaviour, lethargy, loss of consciousness and coma. It is treated by suramin, melarsoprol, pentamidine and eflornithine.
T. brucei life cycle:
Tsetse fly stages: fly bites infected individual, ingesting bloodstream trypomastigotes. Bloodstream trypomastigotes transform into procyclic trypomastigotes in the midgut of the tsetse fly. Procyclic trypomastigotes multiply by binary fission. The procyclic trypomastigotes leave the midgut and transform into epimastigotes. Epimastigotes multiply in the salivary gland and transform into metacyclic trypomastigotes. The infected tsetse fly takes a blood meal, injecting metacyclic trypomastigotes into a new host human.
Human stages: Injected trypomastigotes transform into bloodstream trypomastigotes which are carried to other sites. They multiply by binary fission in various body fluids eg blood, lymph and spinal fluid.
Trypanosoma cruzi causes Chagas’ disease, aka American trypanosomiasis. It’s transmitted by bite and defecation of the Triatomine bug. The parasite affects the heart, gastrointestinal tract and CNS. Occurs in Latin American countries and is diagnosed by microscopy or serology. It’s treated with nifurtimox (Lampit) and benznidazole (Radanil).
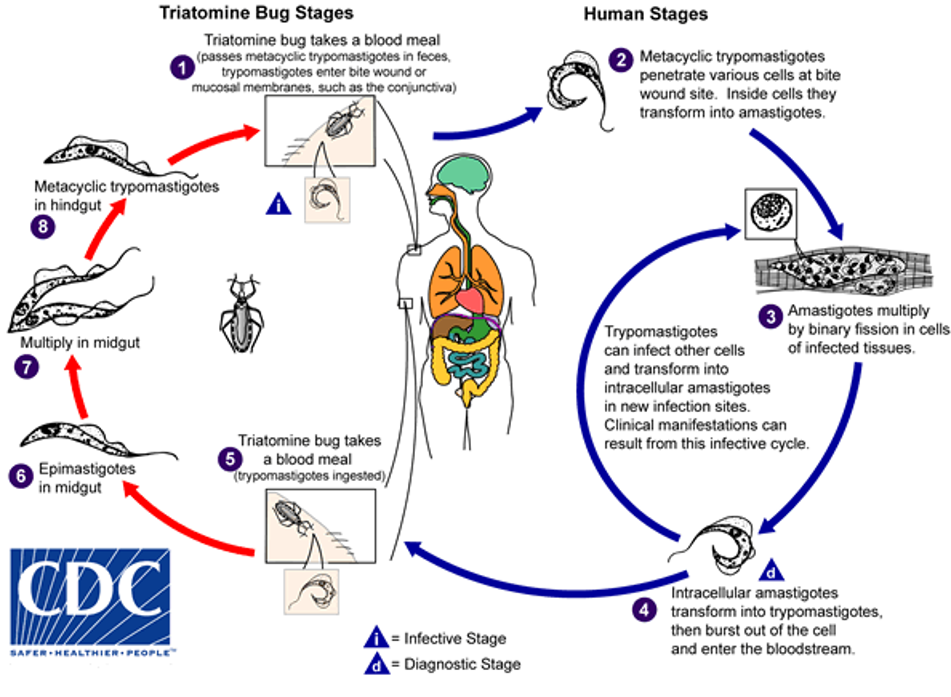
Life cycle:
In triatomine bug: bug takes blood meal from infected individual, ingesting trypomastigotes. Trypomastigotes differentiate into epimastigotes in the midgut and multiply. In the hindgut they transform into metacyclic trypomastigotes. The bug bites a new human host, passes metacyclic trypomastigotes in its faeces and trypomastigotes enter the bite wound or mucosal membranes eg conjuctiva.
In humans: metacyclic trypomastigotes penetrate various cells at the bite wound site. Transform into amastigotes inside the cells. Amastigotes multiply in infected tissue cells by binary fission. The intracellular amastigotes transform into trypomastigotes then burst out of the cell and enter the bloodstream. Trypomastigotes can infect other cells and transform into intracellular amastigotes in new infection sites. Clinical manifestations can arise from this infective cycle.