Rates of Reaction and Energy Change
The rate of reaction
Describes how rapidly the reactants are consumed or the product is formed.
Collision Theory
It explains why different reactions occur at different rates and suggests ways to change the rate of a reaction.
Collision theory states that occurs when particles collide and rates of reaction are increased when the frequency and/or energy of collisions is increased to break bonds.
The factors affecting the number of successful collisions are:
Number of particles per unit volume:
more particles in a given volume increases the number of successful collisions
Frequency of collisions:
greater number of collisions per second → increase in the number of collisions
Activation energy:
The minimum amount of energy that particles must collide with to react.
The more the collision that has energy more than the activation energy → more successful collisions
Kinetic energy of the particles:
The greater the kinetic energy of particles
The more collision will have energy more than the activation energy
More frequent collisions
More successful collisions
Factors affecting rate of reaction:
Temperature at which reaction is carried out.
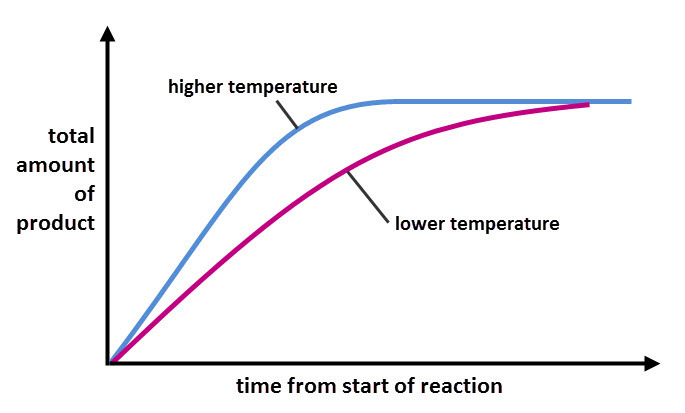
Increase in temperature → increases the rate of reaction.
Increases the rate of reaction because
Particles have more kinetic energy
Collisions occur more frequently and they are harder
Increased successful collisions per second.
The concentration of the reactants in solution (more particles in the same volume).
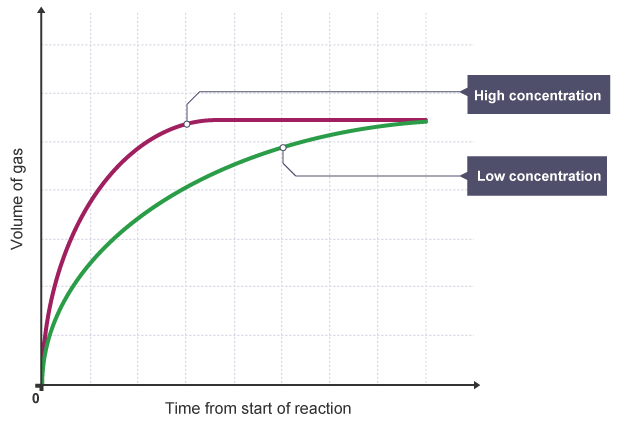
Increase in concentration → increases the rate of reaction.
Increases the rate of reaction as it increases the number of particles in a given volume and so increases the frequency of successful collisions per second
The surface area to volume ratio of solids.
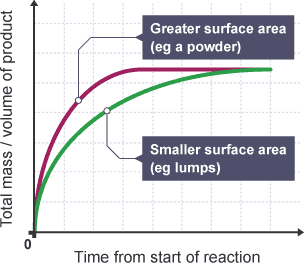
Higher the surface area to volume ratio → increases the rate of reaction.
Particles are closer together, so there are more frequent successful collisions per second.
The pressure of reacting gases.
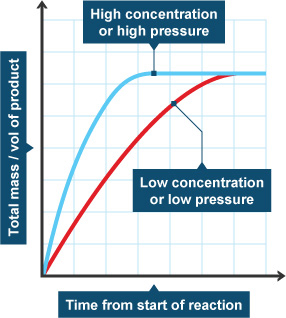
More pressure -> increases the rate of reaction.
increases the rate of reaction as it increases the number of particles in a given volume, so successful collisions are more frequent per second.
Catalyst
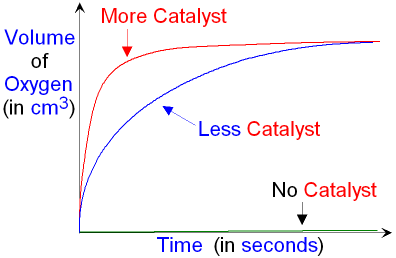
Catalysts are substances that speed up the rate of a reaction without altering the products of the reaction, being unchanged chemically and in mass at the end of the reaction.
They lower the activation energy.
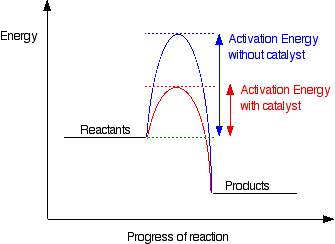
Enzymes are biological catalysts made from protein and enzymes are used in the production of alcoholic drinks.
They can work in lower temperatures
Core Practical:
We investigate the effects of changing the conditions of a reaction on the rates of chemical reactions by
Measuring the production of a gas
Equation:
Marble chips(calcium carbonate) + hydrochloric acid → calcium chloride + water+ carbon dioxide
CaCO_2 +2HCl → CaCl_2 + H_2O +CO_2
The rate of reaction can be measured by the amount of gas produced in the reaction.
The aim of the experiment is to see the effect of surface area on the rate of reaction.
Gas is produced in a reaction, can be trapped, and its volume is measured over time.
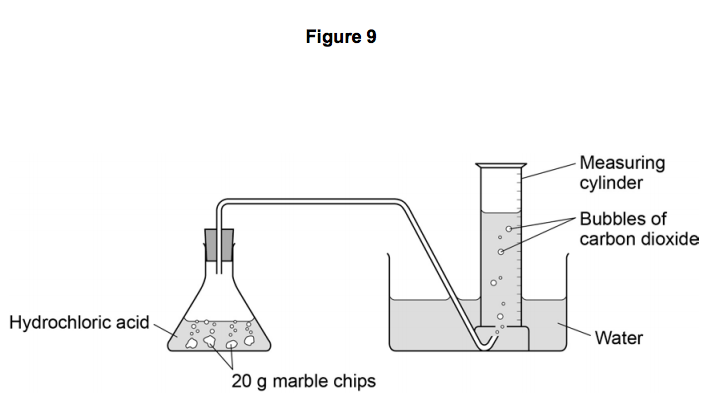
Measure the volume of CO2 using a gas syringe or an upside-down measuring cylinder or burette.
Record the total volume of CO2 collected at regular intervals and plot a graph.
Use the formula: volume of gas/time taken to measure the rate of reaction.
Repeat with different forms of calcium carbonate
Result: Increase in the surface area of the marble chip, the rate of reaction will increase.
Observing a colour change
Equation: Sodium thiosulfate + Hydrochloric acid 🡪 Sodium chloride Water + Sulfur + Sulfur dioxide
Na_2SO_2O_3 + HCl → NaCl + H_2O +S + SO_2
The rate of reaction can be measured by the amount of time taken for the colour to change.
The experiment aims to see the effect of the concentration of Sodium thiosulfate on the rate of reaction.
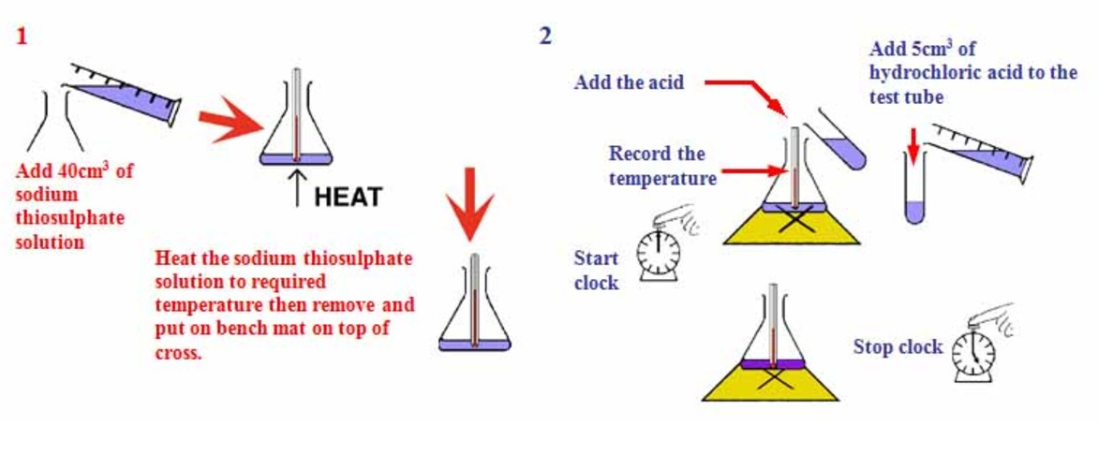
Measure 50 cm3 of sodium thiosulfate solution into a flask
Measure 5 cm3 of dilute hydrochloric acid into a measuring cylinder
Draw a cross on a piece of paper and put it underneath the flask
Add the acid into the flask and immediately start the stopwatch
Look down at the cross from above and stop the stopwatch when the cross can no longer be seen
Repeat using different concentrations of sodium thiosulfate solution (mix different volumes of sodium thiosulfate solution with water to dilute it).
Result: increase in the concentration of a solution, the rate of reaction will increase.
Rate Graphs
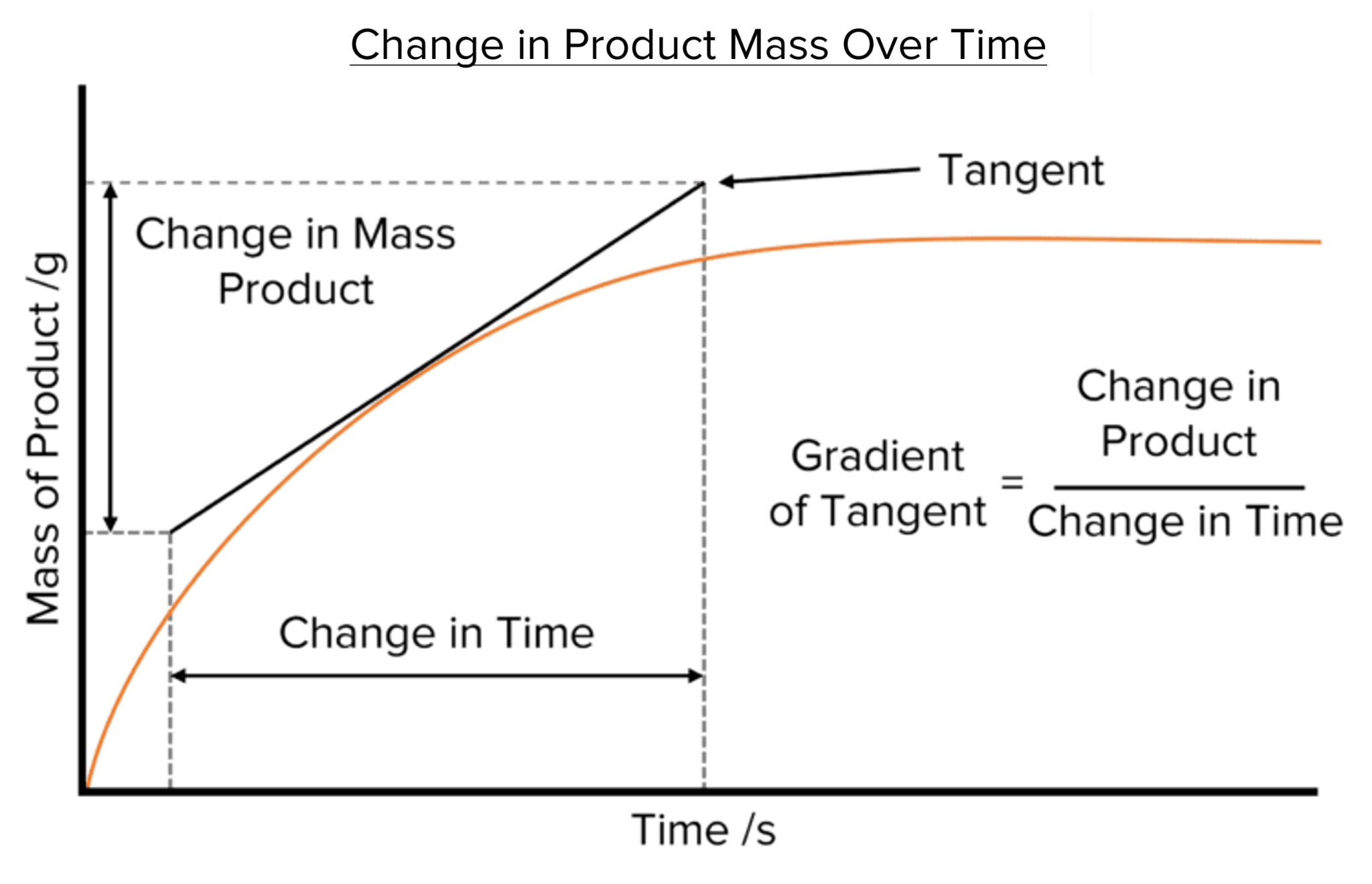
Graphs can measure the rate of reactions by plotting the product/reactant against time.
The gradient of the graph will be the rate of reaction.
The steeper the line, the faster the rate of reaction.